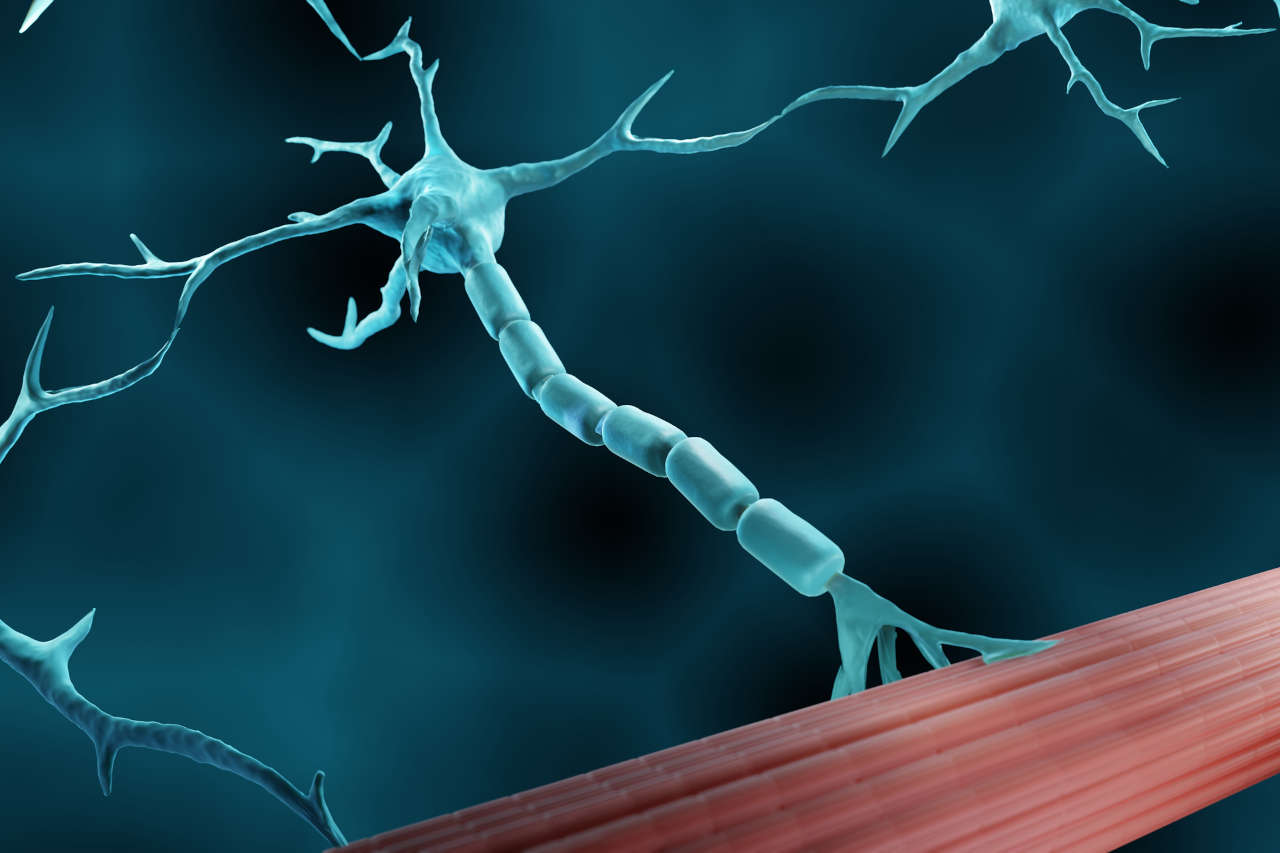
Lambert-Eaton syndrome (LEMS) is a rare disorder that occurs as a result of an immune system attack on the neuromuscular junction (a point where nerves send signals to your muscles). The damage at the neuromuscular junction causes impaired nerve signal transmission to the muscles, which results in gradual muscle weakness and a range of other symptoms.
Speak to a Specialist
About Copay AssistanceThis condition is often referred to as Lambert-Eaton myasthenic syndrome due to the similarities of its symptoms to myasthenia gravis (a type of neuromuscular junction disorder).
The prevalence of Lambert-Eaton syndrome is 46 times lower than myasthenia gravis, and it mostly affects men rather than women.
So, if you or your loved one is recently diagnosed with LEMS, it’s important for you to understand this neurological condition in order to manage it properly.
This article provides a basic understanding of LEMS, including its underlying cause, common signs and symptoms, and various treatment strategies to help you manage this condition.
Basics of Lambert-Eaton Syndrome
Imagine you want to do a certain activity that requires muscle function. Your nervous system (brain) sends signals through nerves to your muscles to perform that action. These nerves release a chemical called acetylcholine, which tells your muscle fibers to contract. The release of acetylcholine is primarily facilitated by the voltage-gated calcium channels present at the end of nerve endings. So, when these channels are activated during nerve signal transmission, it stimulates the release of acetylcholine that binds to its receptors on muscle fiber and causes muscle contraction.
However, in people with Lambert-Eaton syndrome, their immune system produces auto-antibodies that block calcium channels on nerve endings. This blockage causes less acetylcholine release, which is insufficient to cause normal muscle contraction, and as a result, people with LEMS experience muscle weakness and fatigue.
The disease is named after Edward Lambert and Lee Eaton, two neurologists who first described the symptoms of myasthenic syndrome in the 1950s.
Underlying Cause of LEMS
Researchers believe the immune system is the driving factor behind the onset of LEMS. Moreover, in 50% to 60% of the cases, this disease is also found to be highly associated with certain types of cancers, in particular small cell lung cancer (SCLC).
It is theorized that when the immune system fights cancer cells, it also mistakenly attacks the nerve endings since cancer cells contain some of the same proteins that are also present in nerve endings. This mixup is coined “paraneoplastic syndrome,” which can manifest as LEMS.
However, not all cancers cause LEMS. The trigger for LEMS without cancer is currently unknown; however, it is theorized genes linked to autoimmunity may be the cause. Those diagnosed with LEMS without cancer are also much younger, with an average onset of 35 years of age.
Common Signs and Symptoms of LEMS
The onset of LEMS symptoms is gradual and usually develops over weeks or months. However, in the case of patients with small cell lung cancer, the progression of the LEMS symptoms could be rapid.
The common symptom of LEMS is proximal muscle weakness, and the distribution of the muscle weakness begins from the lower limbs (upper legs and hips) and moves upward to the shoulder muscles.

People with LEMS may experience difficulty walking, climbing stairs, and getting out of chairs due to weak hip muscles. With time, the muscle weakness temporarily improves after repetitive muscle activity, which is a unique feature of this condition.
Other LEMS symptoms include:
- Tingling sensation in the hands or feet
- Muscle stiffness & aching
- Fatigue
- Difficulty walking
- Oculobulbar weakness, which is observed in about 70% of patients. Ocular weakness includes ptosis and diplopia.
- Dysphagia (trouble swallowing)
- Dysarthria (trouble speaking)
- Respiratory failure (rare but may occur in the advanced stage of LEMS)
Autonomic dysfunction is also a prevailing concern in LEMS and is observed in about 80% to 96% of patients. The most reported symptoms are dry mouth, constipation, and erectile dysfunction.
Can IVIG help?
Free IVIG Treatment InfoDiagnosis and Tests for LEMS
Neurologists conduct a combination of tests, including a physical evaluation. The neurologist will also consider a patient’s medical and medication history and observe the symptoms. Other medical tests include:
Blood test
This test checks for the presence of autoantibodies in the patient’s circulatory system. Approximately 85% to 95% of individuals with LEMS have these autoantibodies in their blood.
Electromyography
This test checks how your nerves and muscles are working.
Computed tomography (CT), magnetic resonance imaging (MRI) or X-ray
CTs, MRIs, and X-rays are used to check underlying malignancies, such as the presence of small-cell lung cancer (SCLC).
In some cases, LEMS may appear 2 to 3 years earlier before cancer does, and if a person is diagnosed with LEMS, healthcare providers recommend cancer screenings every 3 to 6 months for at least 2 years.
Treatment Options for Lambert-Eaton Syndrome
Even though there is no known cure for LEMS, certain medications and therapy approaches can help patients manage their symptoms. The main goal of the treatment is to treat the underlying cause, such as cancer, and to manage symptoms of LEMS. Therefore, if you have LEMS associated with a cancer like SCLC, treating the underlying cause (cancer) first can help treat LEMS.
Healthcare providers recommend a combination of surgery, chemotherapy, and radiation therapy based on the type of cancer a patient has.
In the case of LEMS treatment, a medication designed to reduce the LEMS symptoms and immune-modulating therapies that suppress the immune response/immune attack on your nerves are typically used.
The most effective medications that are recommended as a first-line treatment for reducing the symptoms of LEMS include:
- Amifampridine (Firdapse):. This drug increases the release of acetylcholine and thus improves the transmission of the signals to your muscles. The recommended starting dosage of Firdapse for adults is 15 to 30 mg per day, divided into three to four doses. Doses may be increased by your healthcare provider to a maximum of 80 mg/day.
- Guanidine: This medicine also increases the acetylcholine release and improves muscle contractions. The recommended starting dose of Guanidine is 15 to 30 mg/kg/day divided into three to four doses. Doses may be increased based on tolerability to a maximum of 35 mg/kg/day.
- Pyridostigmine (Mestinon): This medication works by preventing the breakdown of acetylcholine at the junction where your nerves meet your muscles. By increasing the amount of acetylcholine available, it may help increase muscle strength. Mestinon for this indication is considered off-label, meaning it is not approved by the FDA for this use. Mestinon dosing for LEMS varies as the range is wide and based on tolerability. Your healthcare provider will prescribe the appropriate dose.
Sometimes, when medication does not work well, and the patient continues to have an enduring weakness, healthcare providers recommend immune-modulating or immunosuppressing therapies that suppress the hyperactive immune response. These therapies include:
- Immunoglobulin therapy (IVIG), a procedure in which antibodies are injected into your body that temporarily stop the immune system from attacking your nerves.
- Plasmapheresis, a procedure to filter out autoantibodies from your body, thus preventing the damage of nerves.
- Immunosuppressant drugs to reduce the activity of your immune system.
Besides medication and immunosuppressive therapies, a healthy lifestyle can help to prevent the onset of LEMS. Since, in 50% of cases, LEMS onset is associated with lung cancer, avoiding tobacco products can help prevent cancer.
Prognosis of Lambert-Eaton Syndrome
As long as the underlying condition is promptly treated, the prognosis for LEMS is favorable. Immunosuppressive treatments and drugs generally had a positive effect on LEMS patients, increasing their chances of survival and improving their quality of life.
After diagnosis, 85% of patients regain independence in daily activities within a year of treatment.
REFERENCES:
- Pascuzzi, R. M., & Bodkin, C. L. (2022). Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome: New Developments in Diagnosis and Treatment. Neuropsychiatric Disease and Treatment, 18, 3001-3022. https://doi.org/10.2147/NDT.S296714
- Kesner, V. G., Oh, S. J., Dimachkie, M. M., & Barohn, R. J. (2018). Lambert-Eaton myasthenic syndrome. Neurologic clinics, 36(2), 379-394. https://doi.org/10.1016/j.ncl.2018.01.008
- Titulaer, M. J., Lang, B., & Verschuuren, J. J. (2011). Lambert–Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. The Lancet Neurology, 10(12), 1098-1107. https://doi.org/10.1016/S1474-4422(11)70245-9
- Sanders, D. B. (2003). Lambert-Eaton Myasthenic Syndrome. Annals of the New York Academy of Sciences, 998(1), 500-508. https://doi.org/10.1196/annals.1254.065
- Harper, C. M., & Lennon, V. A. (2018). Lambert-Eaton syndrome. Myasthenia Gravis and Related Disorders, 221-237. https://doi.org/10.1007/978-3-319-73585-6_14
- Merino-Ramírez, M. Á., & Bolton, C. F. (2016). Review of the diagnostic challenges of Lambert–Eaton syndrome revealed through three case reports. Canadian Journal of Neurological Sciences, 43(5), 635-647.
- Hülsbrink, R., & Hashemolhosseini, S. (2014). Lambert–Eaton myasthenic syndrome – Diagnosis, pathogenesis and therapy. Clinical Neurophysiology, 125(12), 2328-2336. https://doi.org/10.1016/j.clinph.2014.06.031
- Ivanovski, T., & Miralles, F. (2019). Lambert-Eaton Myasthenic syndrome: early diagnosis is key. Degenerative Neurological and Neuromuscular Disease, 27-37. https://doi.org/10.2147/DNND.S192588
- Gilhus, N. E. (2011). Lambert-eaton myasthenic syndrome; pathogenesis, diagnosis, and therapy. Autoimmune Diseases, 2011. https://doi.org/10.4061/2011/973808
- McEvoy, K. M., Windebank, A. J., Daube, J. R., & Low, P. A. (1989). 3,4-Diaminopyridine in the treatment of Lambert–Eaton myasthenic syndrome. The New England Journal of Medicine, 321(23), 1567–1571. https://doi.org/10.1056/nejm198912073212303













