What Is Pegfilgratism (Neulasta)?
Pegfilgrastim (pronounced [peg fil GRA stim]), which is also known by the brand name, Neulasta, is a medication that helps your body make more white blood cells. It’s used to raise the white blood cell count in people who receive certain chemotherapy or are exposed to high doses of radiation, which can both lower the white blood cell count. Neulasta was the first medication produced based on the active element pegfilgrastim. Pegfilgrastim is not chemotherapy. Clinics may still have special safety rules for handling any medication—follow your clinic’s rules. Several different biosimilars based on Neulasta have been produced to date. Common U.S. biosimilars include Fulphila, Udenyca, Ziextenzo, Nyvepria, Stimufend, and Fylnetra.
Speak to a Chemo Specialist
What Is Neutropenia?
Neutropenia occurs when you have too few neutrophils, a type of white blood cell that helps our bodies fight off infections. Having neutropenia indicates a higher risk of developing serious bacterial and fungal infections. Neutropenia is a very common side effect of cancer patients undergoing chemotherapy.
Febrile neutropenia is a more serious condition where the neutrophil count drops to a dangerously low level where there are not enough white blood cells to fight off infections. This could potentially lead to life-threatening infections.
Mechanism of Action
Pegfilgrastim binds (attaches) to the granulocyte colony-stimulating factor (G-CSF) receptor to stimulate the bone marrow to make and release more infection-fighting neutrophils.
Speak to a Specialist About Copay Assistance
How is Neulasta Used?
Available Formulations
Neulasta is given as an injection under the skin. It is available as a prefilled syringe and as the Onpro on-body injector (OBI) that gives the dose later. Neulasta injection is available as a single-use prefilled syringe for subcutaneous injection at a dose of 6 mg/0.6 ml. (Children and adults with very low body weight may get different doses—your care team will guide this.)
Subcutaneous Injection
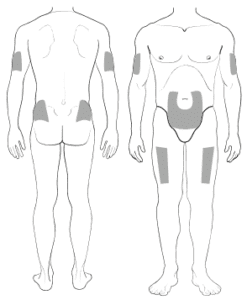
You can inject into the stomach (at least 2 inches from the belly button), front of the thighs, upper outer arms, or upper outer buttocks. Don’t use spots that are sore, red, bruised, scarred, or hard. Don’t rub the injection site after the dose is given. The needle cap has dry natural rubber (a kind of latex). If you’re allergic to latex, don’t handle the capped syringe. You should not attempt to give yourself a subcutaneous injection until you have received the appropriate training from your healthcare provider.
Specialized Skin Patch (On-Body Injector, OBI)
The Onpro device is set to give your dose about 27 hours after it’s put on, which is at least 24 hours after chemo. This is convenient for many patients because it saves them a trip to the doctor’s office the day after receiving chemotherapy.
If the device beeps, flashes, or you think it didn’t give the full dose, call your clinic right away. They may give you a replacement shot.
Missed Dose
If you miss a dose, contact your physician immediately to reschedule. If the OBI malfunctions and fails to deliver medication as scheduled, consult your physician immediately to schedule for the administration of a manual injection as soon as possible.
Storage
Store Neulasta in the refrigerator and avoid freezing. If frozen, thaw in the refrigerator before use. For the prefilled syringe, take it out of the fridge for about 30 minutes before the shot. Throw it away if it has stayed at room temperature for more than 48 hours. Do not shake the vials, and keep them protected from light by storing them in their original containers. The OBI kit should be kept refrigerated until 30 minutes prior to use and should not be left at room temperature for more than 12 hours.
Disposal
Dispose of Neulasta if it has been left at room temperature for more than 48 hours for the syringe or 12 hours for the OBI kit. Dispose of Neulasta if it has been frozen more than once. After injection, dispose of any unused Neulasta left in the prefilled syringe. Be sure to dispose of the pre-filled syringes in an FDA-cleared sharps disposal container (not in the household trash).
Get Chemotherapy Copay Assistance
Chemotherapy Financial AssistanceWhat To Avoid While Taking Pegfilgrastim
While on pegfilgrastim therapy, you must follow some precautions. Always tell your physician about any medication you are already taking. Do not take any drug or medicine (even herbals or over-the-counter medications) without prior consent from your physician or pharmacist, as they may have interactions with Neulasta. Avoid taking Neulasta within 14 days before or 24 hours after having chemotherapy.
Pregnancy and Neulasta
Pregnant women, women who are planning to become pregnant, and breastfeeding women must consult a physician prior to initiating Neulasta. There isn’t much information on pregnancy or breastfeeding. Talk with your provider to discuss the medication’s risks and benefits for you and your baby.
Side Effects
As with any other medication, you may encounter side effects while taking pegfilgrastim. A few things to keep in mind are:
- You may not have all the side effects listed below. Many people may experience little to no side effects.
- The severity of side effects may vary from person to person, so do not compare your side effects with other people’s experiences.
- Most of the side effects will improve when therapy is discontinued.
- Do not hide any symptoms; when you feel any discomfort, do not hesitate to tell your physician or pharmacist about it.
Note: The side effects listed below are not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Some of the more serious side effects of Neulasta are:
Injection-Site Pain
Most needles are painful, but Neulasta is especially known for causing some pain in the injection site, even for a while after the injection is complete. This is also often accompanied by mild bruising, swelling, or redness at the injection site.
Injection site pain can be controlled using over-the-counter painkillers such as Tylenol or Motrin, but usually, it resolves on its own within a few days. Consult your doctor or pharmacist prior to starting any new over-the-counter therapies.
Bone and Muscle Aches
This is the most common side effect of Neulasta since it stimulates the bone marrow (a spongy substance found inside of bone), causing it to swell and cause potential bone pain. It commonly starts about 1 to 2 days after receiving a Neulasta dose and can last up to a week, depending on the person.
Bone or muscle pains can be controlled using over-the-counter painkillers such as Tylenol or Motrin. Consult your doctor or pharmacist before starting any new over-the-counter therapies. Some people try an antihistamine (like loratadine) for bone pain. Ask your provider first to see if this is right for you. Keep in mind that it doesn’t work well for everyone.
Consult a Chemotherapy Specialist
Get Chemotherapy Treatment AssistanceThrombocytopenia, Leukocytosis
Your provider may check your blood cell counts. Sometimes white blood cells get very high, or platelets get low.
Lung Problem Called Acute Respiratory Distress Syndrome (ARDS)
Contact a physician or get emergency medical help right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing.
Kidney Injury
Kidney swelling (glomerulonephritis) can happen. Call your provider if you have puffy face/ankles, blood in urine, or are urinating less.
Capillary Leak Syndrome (CLS)
Neulasta can cause fluid to leak from blood vessels into your body’s tissues. CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency medical help right away if you develop any of the following symptoms: swelling or puffiness, urinating less often, trouble breathing, swelling of the abdominal area, and feeling of fullness, dizziness, or faintness.
Inflammation of Blood Vessels
Aortitis (swelling of a big artery) can happen. Call your provider for fever, tiredness, or deep belly/back pain.
Rupture of the Spleen
A ruptured spleen is one of the most severe side effects of Neulasta. The spleen may become enlarged and may possibly rupture, which can be fatal. Call your doctor right away if you have pain in the upper left stomach (abdomen) or your left shoulder.
Note: Serious allergic reactions can happen—rash, wheeze, face swelling, or trouble breathing, call 911. People with sickle cell disease can have a severe crisis—get help right away if you have new pain or trouble breathing.
Less severe, milder side effects include bone pain, arm/leg pain, tiredness, headache, nausea, and redness at the injection site.
Less severe side effects can include:
- Sweating
- Dark Urine
Get Chemotherapy Copay Assistance
Chemotherapy Financial AssistancePrecautions
Unless approved by your physician, pegfilgrastim is generally not recommended for:
- Patients who have an allergy to human granulocyte colony-stimulating factors (CFS) such as filgrastim or pegfilgrastim products. Allergic reactions can cause a rash over the whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using Neulasta, and call your doctor or get emergency help right away.
- Patients with sickle cell disorder. Using Neulasta can lead to a condition called sickle cell crisis, which may be life-threatening and require discontinuation of Neulasta.
- Patients receiving radiation therapy. In some people with breast or lung cancer, using pegfilgrastim with chemo and/or radiation has been linked to a higher risk of certain blood cancers (MDS/AML). Your team will watch for signs.
FAQs
Is Neulasta a chemo agent?
No, it is not a chemotherapy agent. Instead, it helps treat a major side effect of chemotherapy called neutropenia (low white blood cell count). Chemotherapy drugs are directed at combating your cancer cells, while Neulasta is designed to combat the after-effects of a chemotherapy session.
How is Neulasta taken?
Neulasta is only available as an injection. It can be injected subcutaneously (under the skin) or as a specialized skin patch (also known as an on-body injector [OBI] or Neulasta OnPro) programmed by a healthcare provider. You should not attempt to give yourself a subcutaneous injection until you have received the appropriate training from your healthcare provider. Make sure your dose is at least 24 hours after chemo and not within the 14 days before your next chemo.
REFERENCES:
Burris, H. A., Belani, C. P., Kaufman, P. A., Gordon, A. N., Schwartzberg, L. S., Paroly, W. S., Shahin, S., Dreiling, L., & Saven, A. (2016, September 21). Pegfilgrastim on the Same Day Versus Next Day of Chemotherapy in Patients With Breast Cancer, Non–Small-Cell Lung Cancer, Ovarian Cancer, and Non-Hodgkin’s Lymphoma: Results of Four Multicenter, Double-Blind, Randomized Phase II Studies. Journal of Oncology Practice. 2010 6:3, 133-140. Retrieved November 5, 2021, from https://ascopubs.org/doi/10.1200/JOP.091094
Cancer Statistics. (2020, September 25). National Cancer Institute. Retrieved November 5, 2021, from https://www.cancer.gov/about-cancer/understanding/statistics
FastStats. (2021, October 19). Leading Causes of Death. Retrieved November 5, 2021, from https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
Highlights of Prescribing Information. (2019, April). Food and Drug Administration (FDA). Retrieved November 5, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125031s198lbl.pdf
Instructions for Use Neulasta (Pegfilgrastim). (2016, December). Amgen – Neulasta. Retrieved November 5, 2021, from https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/neulasta/neulasta_pfs_ifu.ashx
Neulasta® (pegfilgrastim) Onpro®. (2021). Resources by Amgen. Retrieved November 5, 2021, from https://www.neulasta.com/resources/
Neulasta: Drug Information. (2018, April 30). Breastcancer.Org. Retrieved November 5, 2021, from https://www.breastcancer.org/treatment/druglist/neulasta
Neulasta: Uses, dosage, side effects, warnings. Drugs.com. (n.d.). Retrieved November 4, 2021, from https://www.drugs.com/Neulasta.html.
Pegfilgrastim (Subcutaneous Route) Side Effects – Mayo Clinic. (2021, February 1). Mayo Clinic. Retrieved November 5, 2021, from https://www.mayoclinic.org/drugs-supplements/pegfilgrastim-subcutaneous-route/side-effects/drg-20066866?p=1
Pegfilgrastim. In: Lexi-drugs online [database on the Internet]. Hudson (OH): Lexicomp, Inc.; 2016 [updated 329 Oct 2021; cited 4 Nov 2021]. Available from: http://online.lexi.com
Pegfilgrastim. In: In Depth Answers [database on the Internet]. Greenwood Village (CO): IBM Corporation; 2017 [cited 2021 Nov 4]. Available from: www.micromedexsolutions.com
Weiser, P. P. (2021, May 6). All About Neulasta. Healthline. Retrieved November 5, 2021, from https://www.healthline.com/health/drugs/neulasta#what-it-is