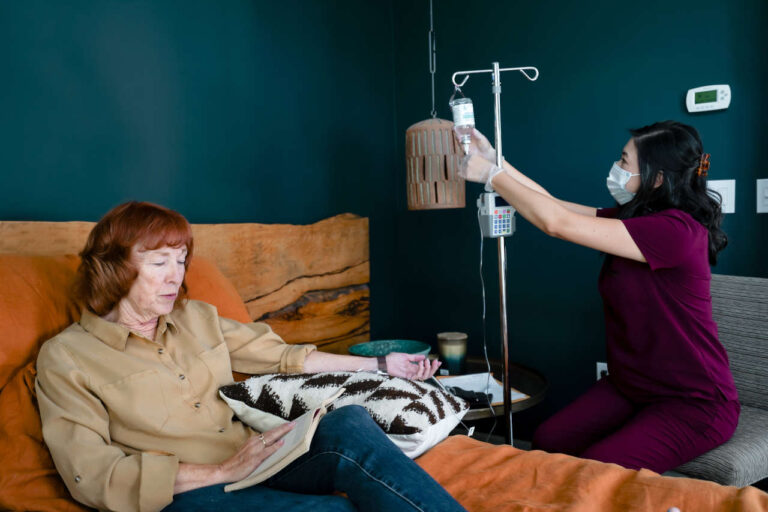
Though not recommended for routine use, IVIG for bone marrow transplant may be an option for specific individuals with severe immunodeficiency and recurrent infections. Learn about the benefits of IVIG for specific populations, common side effects, and more.
Consult an IVIG Specialist
A Quick Overview of Bone Marrow Transplant
A bone marrow transplant is an important treatment option for certain cancers.
In this procedure, healthy cells (which will eventually become blood cells) are administered into the body. These cells — stem cells — replace the diseased or damaged bone marrow. Several factors can damage or weaken the bone marrow, such as immune disorders, radiation, or chemotherapy.
Other names for this procedure are:
- Hematopoietic stem cell transplant
- Peripheral blood stem cell transplant
- Umbilical cord blood transplant
There are two sources of stem cells:
- Your own body (autologous transplantation)
- A donor (allogenic transplantation)
Your hematologist may recommend a bone marrow transplant to:
- Help your body produce enough healthy blood cells.
- Treat cancer along with high-dose chemotherapy or radiation.
- Increase the levels of stem cells, which can become immune cells that destroy cancer cells.
A bone marrow transplant can also benefit people with noncancerous diseases like immune deficiency disorders and certain inherited blood disorders.
According to the Center for International Blood and Marrow Transplant Research (CIBMTR), over 8,000 individuals received stem cells from donors in the U.S. in 2016 [1].
What Are the Potential Benefits of IVIG for Bone Marrow Transplant?
IVIG may benefit select patients undergoing a bone marrow transplant. Early studies suggest IVIG may help prevent infections, manage post-transplant autoimmune disorders, and reduce the risk of graft-versus-host disease (GVHD).
GVHD happens when the donor stem cells attack and destroy healthy cells in the recipient (host).
While older studies found a promising role of IVIG for bone marrow transplant, newer studies and extensive reviews have failed to find any clear benefit of IVIG, especially with routine use for the general transplant patients.
For example, a 2008 review of 30 trials including over 4,000 individuals undergoing bone marrow transplantation found no benefits of IVIG in improving survival or preventing infections [2].
IVIG for Bone Marrow Transplant: Potential Benefits for Specific Populations
Though convincing data are unavailable, IVIG may be considered in specific populations.
IVIG for Cytomegalovirus (CMV) Pneumonia After a Bone Marrow Transplant
The recurrence of a CMV infection is a potentially life-threatening complication in which the dormant CMV becomes active following a transplant, often an allogenic transplant. Complications of CMV reactivation can include pneumonia, inflammation in the digestive tract, and vision problems.
According to a 2014 study, IVIG 0.5 g/kg weekly for the first 3 months after a bone marrow transplant from a donor may help reduce [3]:
- Cytomegalovirus (CMV) load
- The need for anti-CMV therapy
There’s no strong evidence to support the use of IVIG with standard antiviral therapy for CMV pneumonia after bone marrow transplantation.
Nevertheless, some experts recommend combining IVIG with anti-CMV medication. In such cases, the dose of IVIG is 150 mg/kg every other day for 14 days. This is followed by IVIG 150 mg/kg every week for the next 4 weeks, administered with anti-CMV medication [4].
Get IVIG Copay Assistance
IVIG in Patients With Hypogammaglobulinemia
IVIG for bone marrow transplant may also be an option in patients with extremely low antibody levels (severe hypogammaglobulinemia; IgG <4 g/L) [5]. Hypogammaglobulinemia affects one in four patients after a bone marrow transplant involving a donor [6].
IVIG for Bone Marrow Transplant: What Do Current Medical Guidelines Say?
Current guidelines don’t recommend the use of IVIG to prevent infections in all transplant patients. Nonetheless, IVIG therapy may be considered in specific populations or situations, after assessing the risks, benefits, and costs.
For example, according to the Centers for Medicare and Medicaid Services (CMS), IVIG for bone marrow transplant may be an option in individuals 20 years or older during the first 100 days following a transplant. Notably, the CMS doesn’t recommend IVIG in individuals younger than 20 years and those undergoing autologous transplants [7].
IVIG for Bone Marrow Transplant: What Are the Side Effects?
IVIG products are generally well tolerated. Occasionally, severe side effects can occur, including:
- Swelling of the lining of the brain
- Blood clots in the lungs
- Fluid accumulation in the lungs
- Severe, potentially fatal allergic reactions
- Kidney failure or reduced kidney function
- Severe infections
- Early breakdown of red blood cells
Common and mild side effects can include nausea, fever, chills, fatigue, joint aches, headache, and muscle pain.
Frequently Asked Questions
Why is IVIG given to transplant patients?
IVIG is more commonly administered after a bone marrow transplant.
If given before a transplant, IVIG is used in specific cases, such as when a patient is receiving a transplant from someone who is CMV positive, when the patient is CMV negative. CMV-specific IVIG in this instance helps prepare a patient for transplantation by reducing the antibody level in their body. As a result, the recipient’s immune system is less likely to reject the transplanted bone marrow.
When given after a transplant, IVIG can help reduce the risk of infections.
What are the advantages of IVIG treatment after a stem cell transplant?
IVIG after a bone marrow or stem cell transplant can help prevent infections and lower the risk of post-transplant autoimmune conditions.
What is the success rate of IVIG infusions?
IVIG success rates can range from 60% to 80%, depending on the condition being treated, individual response, and the specific IVIG brand.
How does IVIG work in rejection?
IVIG therapy reduces the immune system’s ability to identify and attack the transplanted bone marrow.
Does IVIG therapy improve survival in bone marrow transplant patients?
According to a 2024 study, IVIG use was associated with a decrease in sepsis (an extreme and life-threatening immune response to an infection). However, the researchers failed to find any positive effect of IVIG on survival [8].
REFERENCES:
- Khaddour K, Hana CK, Mewawalla P. Hematopoietic Stem Cell Transplantation. [Updated 2023 May 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536951/#
- Raanani, Pia et al. “Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology vol. 27,5 (2009): 770-81. doi:10.1200/JCO.2008.16.8450
- Yannakou, Costas K., et al. “Intravenous Immunoglobulin Post Allogeneic Stem Cell Transplantation Is Associated With Lower Levels of CMV Reactivation.” Blood, vol. 124, no. 21, Dec. 2014, p. 2482, doi:10.1182/blood.v124.21.2482.2482.
- FRED HUTCHINSON CANCER CENTER, Carpenter, P., Boeckh, M., Cheng, G.-S., Stern, J., & Holmberg, L. (2025). LONG-TERM FOLLOW-UP AFTER HEMATOPOIETIC STEM CELL TRANSPLANT. https://www.fredhutch.org/content/dam/www/research/patient-treatment-and-support/ltfu/LTFU_HSCT_guidelines_physicians.pdf
- Cowan, Juthaporn, et al. “Protocol for Updating a Systematic Review of Randomised Controlled Trials on the Prophylactic Use of Intravenous Immunoglobulin for Patients Undergoing Haematopoietic Stem Cell Transplantation.” BMJ Open, vol. 5, no. 8, Aug. 2015, p. e008316, doi:10.1136/bmjopen-2015-008316.
- Chai, Khai Li et al. “Immunoglobulin replacement to prevent infections in people with haematological malignancies and haematopoietic stem cell transplantation.” The Cochrane Database of Systematic Reviews vol. 2024,3 CD015719. 4 Mar. 2024, doi:10.1002/14651858.CD015719
- LCD – Off-Label Use of Intravenous Immune Globulin (IVIG) (L39314). www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=39314&ver=7.
- Kaya, Ahmet; Berber, İlhami; Kuku, İrfan; Kaya, Emin; Erkurt, Mehmet Ali; Biçim, Soykan1; Arslan, Süleyman; Yağin, Fatma Hilal2. Prophylactic intravenous immunoglobulin use in allogeneic stem cell transplantation; does intravenous immunoglobulin affect survival, sepsis, and engraftment time?. Iraqi Journal of Hematology 13(2):p 202-207, Jul–Dec 2024. | DOI: 10.4103/ijh.ijh_41_24