Hizentra belongs to the class of medications called immunoglobulins. It is used to treat primary immunodeficiency and nerve conditions such as demyelinating polyneuropathy.
Let’s take a closer look at Hizentra, its uses, and side effects.
Speak to a Specialist
About Hizentra Copay AssistanceThe U.S. Food and Drug Administration (FDA) first approved the medication in 2010. Since then, it has gained widespread popularity, becoming the world’s most prescribed immunoglobulin medication for primary immunodeficiency (PI), with over 9.3 million exposures worldwide.
The color of Hizentra can vary from clear and pale yellow to light brown and it is available in pre-filled syringes and vials in various sizes. Your pharmacist and prescriber can help determine the appropriate form to use.
How Does Hizentra Work?
Hizentra’s active ingredient, human immunoglobulin, is a highly purified protein derived from the plasma of healthy individuals. It acts as the body’s defense system to prevent bacterial and viral infections and create a balanced immune system.
Autoimmune diseases happen when the body’s immune system is unable to differentiate your own cells from foreign cells and mistakenly attacks normal cells. Hizentra infusions decrease the immune system’s hyperactivity.
Who Can Use It?
Adults and children over two years old can use Hizentra. Patients using this medication often have nerve disorders and frequent infections. Such disorders include chronic inflammatory demyelinating polyneuropathy (CIDP) and autoimmune system diseases like:
- Hypogammaglobulinemia
- Common variable immunodeficiency (CVID)
- X-linked agammaglobulinemia
- Wiskott-Aldrich syndrome
Speak With a Specialist
Free ConsultationAdministration
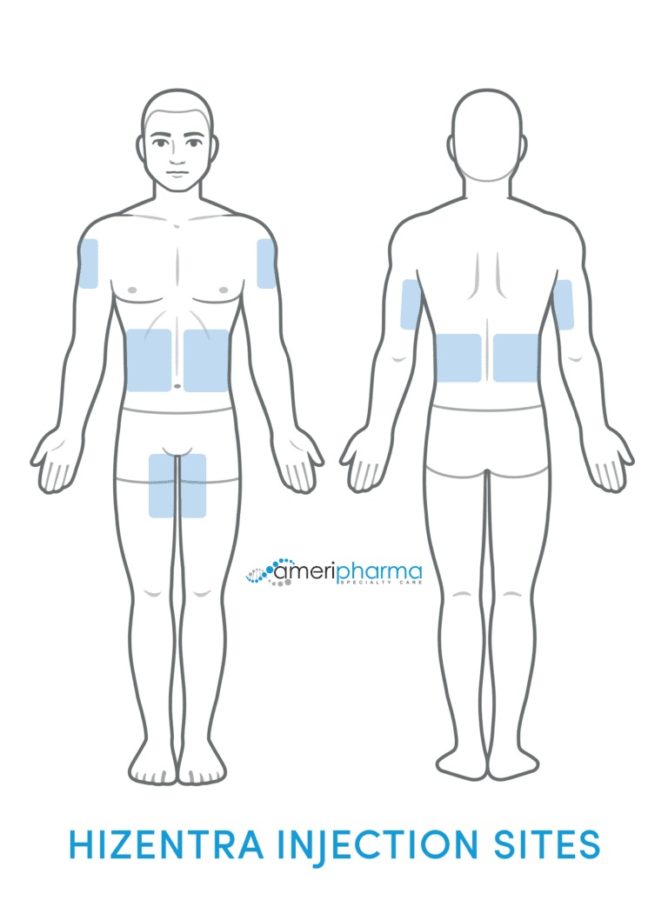
Hizentra must be administered a few times a month according to the doctor’s instructions to decrease symptoms and avoid Hizentra’s side effects. It is administered subcutaneously on the abdomen or thigh through multiple needles positioned under the skin. It can be administered by a healthcare professional or self-administered after proper training.
Use the following tips to avoid adverse effects:
- The doctor or pharmacist will calculate the correct dosage of Hizentra infusion based on the patient’s weight and response to treatment.
- It is not advisable to change the dosage intervals without consulting a physician.
- Patients who miss a dose can contact the pharmacist to modify the dosage plan.
- If a patient overuses the prescribed dosage, they should seek medical help.
- Patients are advised against consuming large doses to achieve quicker results. The consequences may be fatal.
 Other Usage Requirements:
Other Usage Requirements:
- Do not use the medication if it is cloudy, contains particles, or has changed colors.
- Do not use the medication if the protective cap is missing or defective.
- Do not use the medication if the expiration date on the label has passed.
- Do not shake the Hizentra vial or pre-filled syringe.
- Make sure that you do not infuse the drug into your vein.
- Make sure you assemble the supplies and needle set in a clean place.
- Wash your hands before and after every use.
- Discard any unused product and all used disposable supplies after each infusion.
Hizentra Dosing Information
Hizentra available sizes: 5 ml, 10 ml, 20 ml, and 50 ml prefilled syringes and single-dose, ready-to-use vials containing 0.2 grams/ml.
Hizentra is administered as a subcutaneous (under the skin) injection using an infusion pump. The sites of administration are the stomach, thigh, upper arm, or hip. Use a different administration site that’s at least 1 inch away from the previous infusion site. More than one infusion device, and up to 8 infusion sites, can be used at the same time. Each infusion site must be at least 2 inches apart from each other.
Primary Immunodeficiency (PI)
Before using Hizentra, a patient must receive immunoglobulin (Ig) therapy for at least 3 months. Additionally, a serum immunoglobulin G (IgG) level should be drawn to help guide Hizentra dosing.
Dose
The starting Hizentra dose will depend on the previous Ig dose:
- Switching from intravenous Ig (IVIG) to Hizentra: The weekly Hizentra dose is calculated by dividing the previous IVIG dose in grams by the number of weeks between doses, then multiplying by 1.37. For example, if the IVIG dose was 30 grams every 4 weeks, the weekly Hizentra dose would be calculated as follows: 30 divided by 4, then multiplied by 1.37, which equals 10.3 grams (round down to 10 grams) of Hizentra once per week.
- Switching from subcutaneous Ig (SCIG) to Hizentra: The weekly Hizentra dose should be the same as the previous weekly SCIG dose.
Hizentra should be started 1 week after the previous IVIG or SCIG dose, and can be given from once daily to up to once every 14 days, as long as the same weekly dose is maintained.
After 2 to 3 months of receiving Hizentra, the dose can be adjusted based on clinical response and serum IgG levels.
Infusion Rate
For the first infusion, don’t use more than 15 ml per infusion site, and don’t infuse faster than 15 ml/hour. For subsequent infusions, the volume can be increased to 25 ml and the infusion rate can be increased to 25 ml/hour per infusion site.
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Dose
The usual starting dose of Hizentra for CIDP is 0.2 g/kg per week, which can be given in 1 to 2 sessions over 1 to 2 days. If symptoms worsen, the dose can be increased to 0.4 g/kg per week, given in 2 sessions over 1 to 2 days. If symptoms worsen on 0.4 g/kg dose, consider alternative treatment.
Infusion Rate
For the first infusion, don’t use more than a volume of 20 ml per infusion site, and don’t infuse faster than 20 ml/hour. But for subsequent infusions, the volume can be increased to 50 ml and the infusion rate can be increased to 50 ml/hour per infusion site.
Hizentra’s Side Effects
Reactions Around the Injection Site:
- Rash
- Pain
- Redness
- Swelling
- Itching
- Bruising
Other Side Effects:
- Headache
- Flu-like symptoms
- Pain (including pain in the chest, back, joints, arms, and legs)
- Cough
- Nausea and vomiting
- Fatigue
Severe Side Effects
If the following side effects are observed after a Hizentra infusion, tell your doctor immediately or go to the emergency room.

Signs of a Bad Allergic Reaction to Hizentra:
- Hives
- Trouble breathing
- Wheezing
- Dizziness
- Fainting
Signs of a Kidney Problem:
- Reduced urination
- Sudden weight gain
- Swelling in your legs
Signs of a Blood Clot:
- Pain or swelling of an arm or leg with warmth over the affected area.
- Discoloration of the arm or leg.
- Unexplained shortness of breath, chest pain, or discomfort worsening upon deep breathing.
- Unexplained rapid pulse, numbness, or weakness on one side of the body.
Signs of Brain Swelling Called Meningitis:
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light.
Signs of a Blood Problem:
- Brown or red urine
- Fast heart rate
- Yellow skin or eyes
Signs of an Infection:
- Fever over 100ºF
Seek emergency help if you have chest pains or trouble breathing after receiving Hizentra.
The side effects, as mentioned earlier, may occur after prolonged immune globulin therapy. In rare cases, an ulcer may develop at the injection site of the Hizentra infusion from improper administration techniques. Your doctor can adjust the dosage to reduce the risk. Patients can also manage the side effects by taking oral premedications prior to infusion therapy.
Side effects that are not listed above may also occur. In such cases, the patients should seek medical help.
Get Your Hizentra Dose
At Home IVIG InfusionCan Pregnant Women and Breastfeeding Mothers Take Hizentra?
Not enough clinical studies are available to indicate any possible side effects Hizentra may have on pregnant women and nursing mothers. It is unknown whether Hizentra can be dangerous to the fetus or a woman’s reproductive capacity.
Immunoglobulins increasingly cross the placenta after thirty weeks of gestation. The medication should be administered to pregnant women only if it is clearly needed. Even then, administration must be taken with caution and with approval from a doctor.
Notify your doctor if you plan to get pregnant or breastfeed.
How Should Hizentra Be Administered?
Hizentra should only be self-administered after you have received adequate training from either a healthcare professional or a doctor. The infusion is typically carried out in subcutaneous zones like the thigh, lateral hip, belly, and upper arm.
Can the Medication Be Given Intravenously (IV)?
The medication should be infused under your skin only (subcutaneously). DO NOT inject it into a blood vessel (vein or artery).
Hizentra Infusion Rate
The pharmacist will determine the dose and infusion rate of Hizentra based on the patient’s needs and tolerability. The infusion is given via an infusion pump.
How Fast Does Hizentra Work?
The onset of action may vary for each patient, but most will begin to notice a decrease in symptoms 3 to 4 weeks after their initial infusion.
Patients often begin to see results from their treatments by noticing better control of their symptoms or slowing disease progression. During this time, your doctor will closely monitor and track you to see if there are improvements in the symptoms associated with your primary diagnosis.
Infusion times average 1 to 1.5 hours and can be adjusted by the pharmacist if side effects occur or a change in the number of needles used is requested.
Interactions
Drug Interactions
Some medications can impact how Hizentra works in the body and may interfere with each other. As such, it is essential to notify your pharmacist of your medication list and vaccines to avoid Hizentra’s side effects.
The passive transfer of antibodies with immunoglobulin administration may diminish the effects of live virus vaccines such as:
- Measles, Mumps, and Rubella (MMR)
- Varicella (Chickenpox)
It is vital to inform your doctor when using other prescriptions with Hizentra.
Disease Interactions
It is not safe to receive Hizentra infusions when you have the following conditions:
- Allergy to the medication or any of its properties
- Allergy to polysorbate 80
- Blood disease
- Thrombosis
- Dehydration
- Renal disease
- Hyperprolinemia (excessive proline in the body)
Other Contraindications
Hizentra may interact with specific blood tests and provide an inaccurate test result. Patients should inform their doctor that they are taking immunoglobulin before having any blood work done.
 Storage
Storage
When storing:
- Keep the medication away from children and pets.
- Do not use it after its expiration date.
- Hizentra should be stored at room temperature (up to 25°C [77°F]) and is stable for up to 30 months, as indicated by the expiration date.
- Protect the medication from light and heat. Store it in the original carton.
- Do not shake.
- Do not freeze.
How Much Does Hizentra Cost?
Hizentra is expensive. However, the cost of this medication depends on various factors.
- Patient’s insurance status
- Coinsurance regulation
- Individual prescriptions
- Medication dosage
- Other factors
For patients who do not have insurance, the following table will provide an overview of the approximate cost of Hizentra infusions. Note that the prices are for the U.S. only.
| Size | Per Unit | Cost |
|---|---|---|
| 5 ml | $51.23 | $256.15 |
| 10 ml | $50.30 | $503.04 |
| 20 ml | $49.84 | $996.83 |
| 50 ml | $49.56 | $2,478.21 |
Speak to a Specialist
Where Can Patients Obtain Hizentra?
The medication can be obtained from any reputable specialty pharmacy. It may also be purchased over the internet through online specialty pharmacies. However, there are risks to buying online, as you may end up with low-quality or counterfeit Hizentra and experience side effects.
It is recommended to buy from a licensed online pharmacy. AmeriPharma® Specialty is a URAC and ACHC-accredited online pharmacy that dispenses Hizentra and other immunotherapy medications.
Copay Assistance
Copay assistance relief programs are available for patients who cannot afford the cost of Hizentra. These programs are committed to providing timely resources to ensure that patients can initiate immune globulin therapy as soon as possible.
These relief programs reduce financial burdens for patients and the members of their families.
Make sure you contact AmeriPharma® Specialty Pharmacy if you are interested in copay assistance for Hizentra infusions.
REFERENCES:
- http://www.hizentra.com/pi
- http://www.pmnewswire.com/news-releases/hizentra-immune-globulin-subcutaneous-human-20-liquid-label-update-provides-new-dosing-guidelines-for-physicians-allowing-for-greater-flexibility-in-treating-cidp-patients-301280065.html
- http://www.medicines.org.uk/emc/product/4643/xpil/print#gref
- European Medicines Agency. 2018. “An Overview of Hizentra and why it is authorized in the EU.” p.1
- http://www.singlecare.com/prescription/hizentra/what-is
- http://www.drugs.com/drug-interactions/immune-globulin-subcutaneous,hizentra.html
- http://www.drugs.com/price-guide/hizentra
- https://ameripharmaspecialty.com/ig-home-infusion/
- http://www.webmd.com/drugs/2/drug-153823/hizentra-subcutaneous/details
- http://www.nps.org.au/medicine-finder-hizentra-vial
- http://www.nps.org.au/medicine-finder/hizentra-vial#full-pi

 Other Usage Requirements:
Other Usage Requirements: Storage
Storage