Immunoglobulin is a product composed of proteins produced by the body to protect and aid in the fight against infections. These proteins are also known as antibodies. Gammagard is one of the most frequently used immunoglobulin products.
Immunoglobulin products are produced from the blood (plasma) taken from thousands of donors. Through human plasma, antibodies can be isolated from donated blood and used to make this medication. Takeda Pharmaceutical Company manufactures this product, which is also known as immune globulin or under the brand name Gammagard liquid IV. It was initially used to treat and manage Alzheimer’s disease.
It has also been FDA-approved to treat primary immunodeficiency. This is when the body has an insufficient number of antibodies, leading to frequent or recurrent infections. Through immunoglobulin therapy, the antibodies collected from healthy individuals are administered to those who need to replace these missing antibodies. This therapy has been approved for patients two years and older who are diagnosed with this condition.
Gammagard is a 10% IgG solution (100 milligrams/ml), free of sucrose, added sugars, sodium, preservatives, and proline. As a result, this medication is ideal for individuals allergic to these additives.
According to the manufacturer, Gammagard IVIG liquid has been shown to be safe and effective in numerous therapies. This is primarily due to the potential infection screening process that all donors undergo. In addition, all human plasma is collected only at FDA-approved locations.
Get IVIG Copay Assistance
Speak to a SpecialistGammagard Treatment/Therapy

Beta-Amyloid proteins, abbreviated as Aβ, are synthesized throughout the human body. A neurodegenerative process can begin when these proteins aggregate and form plaques in the brain. This process results in memory loss and cognitive impairment, as seen in patients with Alzheimer’s disease. Immune globulins can help decrease the Aβ toxicity in the brain.
According to clinical studies, patients diagnosed with mild Alzheimer’s disease treated with Gammagard liquid have a higher level of Aβ antibodies in their serum and a lower level of clumped Aβ proteins in their brains. This has led to patients’ MMSE (Mini-Mental State Exam) scores stabilizing for over a year and a half.
Another study reported improved changes in the level of human plasma cytokines in patients with mild to moderate Alzheimer’s disease. MRI scans revealed a reduction in the heart’s enlargement.
The FDA has also approved Gammagard IVIG liquid to treat immunodeficiency and autoimmune diseases. Some of these conditions include primary immunodeficiency (PI) and multifocal motor neuropathy (MMN).
PI occurs when the body’s immune system is deficient and does not produce enough antibodies. Immunoglobulin therapy is required to keep patients with PI healthy.
MMN occurs when the immune system attacks the body’s nerves. Gammagard is used to maintain muscle strength in adult patients with MMN.
Your IVIG Treatment Info
Get IVIG Prior AuthorizationGammagard Side Effects
This therapy has some commonly known and potentially serious side effects. These include nausea, vomiting, and diarrhea.
Side effects may vary depending on the diagnosis, route of administration, and tolerability of the therapy, but is overall very well tolerated in many patients. Below are common side effects for patients under treatment for PI and MMN.
PI Common Side Effects
IV Administration:
- Rash or itchy skin
- Chills
- Cough
- Sudden feeling of cold, associated by a rise in temperature (Rigors)
- Fever (Pyrexia)
- Asthma
- Fatigue
- Nausea
- Muscle pain (Myalgia)
- Diarrhea
- Migraine
- Dizziness
- Vomiting
- Joint stiffness (Arthralgia)
- Headache
- Cardiac murmur
- Fluid accumulation or pain in extremities
Subcutaneous Administration:
- Fatigue
- Fever (Pyrexia)
- Nausea
- Asthma
- Ear pain
- Diarrhea
- Migraine
- Vomiting
- Headache
- Increased heart rate
- Upper abdominal pain
- Pain in the extremities
- Infusion site (local) reactions
- Increased systolic blood pressure
MMN Common Side Effects
Common side effects include:
- Nausea
- Headache
- Muscle spasms
- Chest discomfort
- Muscle weakness
- Pain in head or neck (Oropharyngeal pain)
- Pain in the extremities
Contact a medical professional immediately if you are experiencing side effects and have questions.
If you are experiencing severe side effects, seek emergency medical attention. Call 1-800-FDA-1088, or contact your doctor as soon as possible.
The medicine’s package insert contains additional information on the severe side effects of Gammagard IVIG solutions.
Common Injection Site Side Effects
- Mild to moderate pain
- Swelling
- Itchiness
- Bruising
- Redness
- Low-grade fever
- Warmth
The above side effects are mainly noticeable at the injection site. They will usually subside within a few hours following your infusions. However, it is still important to inform your doctor as soon as possible if you are experiencing any side effects.
Common General Side Effects
- Light headache
- Migraine
- Redness or warmth at the injection site
- Fatigue
- Low-grade fever
- Itchiness or rash
- Cough
- Chest pain
- Chills
- Difficulty breathing
- Dizziness
- Nausea and vomiting
- Increased heart rate
- Increased blood pressure
- Upper abdominal pain
- Muscle cramps and weakness
- Sore throat
These side effects are less likely to occur after the first few infusions.
Side Effects of Gammagard Liquid Injection for MMN
The following are some of the side effects of Gammagard liquid injection for MMN or multifocal motor neuropathy. The side effects listed are rare and less likely to occur:
- Severe headache
- Chest pain
- Muscle spasms and weakness
- Breathing difficulty
- Nausea
- Sore throat
- Dizziness
- Redness
- Fever
- Pain in the ears, hands, and feet
Ask a Gammagard IVIG Specialist About Side Effects
Severe Side Effects
The items listed below are severe and rare side effects that may occur while taking Gammagard:
Allergic (Anaphylactic) Reaction
- Hives
- Swelling or tightness in the mouth or throat (wheezing)
- Low blood pressure
- Rapid heart beat
- Trouble breathing
- Dizziness with fainting
Kidney Problems
- Reduced urination caused by kidney failure
- Weight gain
- Swollen legs (which maybe a sign of kidney failure)
Liver Problems
- Yellow skin or eyes
Blood Clots (Thrombosis)
- Pain and swelling of legs and arms
- Brown, red, or dark-colored urine
- Fast heart rate
Heart and Lung Complications
- Chest pain
- Trouble breathing
- Blue lips and extremities
Risk of Infection
- Fever over 100°F
Aseptic Meningitis Syndrome
- Fever
- Chills
- Body aches
- Sensitivity to light
- Loss of appetite
- Vomiting
- Fatigue
- Nausea
- Stiff neck
Hemolysis (Destruction of Red Blood Cells)
- Abnormal paleness of skin
- Yellowing of the skin or eyes (signs of jaundice)
- Dark urine
- Fever
- Weakness
- Dizziness
- Confusion
Transfusion-Related Acute Lung Injury
The following symptoms typically occur within 1 to 6 hours of Gammagard treatment:
- Difficulty breathing or wheezing (signs of pulmonary edema)
- Changes in the color of skin, confusion, cough, fast heart rate, rapid breathing, shortness of breath, slow heart rate, sweating (all of the following are signs of hypoxia)
- Fever
- Transmittable infectious agents (because this drug comes from human plasma, it may carry a risk of transmitting infectious agents)
It is crucial to recognize that not all the warnings or side effects listed in this section are likely to occur with Gammagard IVIG solutions. Contact a medical professional immediately if a patient is experiencing an allergic reaction or severe side effects (call 1-800-FDA-1088, or contact your doctor as soon as possible).
Consider reading Gammagard’s package insert if you want to learn more about these common and severe side effects in greater detail.
Is IVIG Infusion not working?
The Best IVIG Home Infusion | IVIG IndicationsAdverse Reactions

Adverse reactions are effects caused by the administration of medication therapy. The following are some signs of severe adverse reactions that may arise as a result of using Gammagard:
- Difficulty breathing
- Skin rashes
- Kidney failure
- Blood clots in the heart and lung
- Severe headache and fatigue
- Fever
- Chills
- Painful eye movements
- Sensitivity to light
- Nausea and vomiting
- Drowsiness
- Dark-colored urine
- Muscle swelling
If a patient is experiencing signs and symptoms of severe adverse reactions such as those listed in this section, contact emergency services. Dial 1-800-FDA-1088, or notify your doctor immediately.
The package insert for Gammagard SD can be accessed here, which contains additional information on these adverse reactions.
Precautions and Contraindications
Gammagard IVIG liquid is a highly beneficial medication, particularly for those who suffer from immunodeficiency. Each patient should be thoroughly screened for the following before initiating therapy.
- Allergies to immune globulin and blood products
- Selective or severe immunoglobulin A (IgA) deficiency
Gammagard Liquid With Vaccines
Gammagard therapy may impair the immune response and diminish the effectiveness of live vaccines such as MMR or chickenpox. Inform your immunizing physician if you are currently on immune globulin therapy.
Gammagard Liquid in Pregnancy
Gammagard therapy is classified as a pregnancy category C medication. This means there is limited literature on this medication. No animal reproduction or effect on fetal development studies have been done. It is unknown whether it can harm a developing fetus or impair reproductive capacity when administered to a pregnant woman.
After 30 weeks of gestation, immunoglobulins can increasingly cross the placenta from the maternal circulation. The liquid should be used during pregnancy only when clinically indicated.
Gammagard Liquid in Lactating Women
No studies have shown the excretion of Gammagard in breast milk. However, since many medications have the risk of entering breast milk, caution should be used when administering Gammagard IVIG solutions to someone who is breastfeeding.
Understanding the precautions for immune globulin will greatly improve the efficacy of the therapy and help prevent adverse outcomes. Inform your doctor about your medical history and other pertinent health information before the treatment.
Formulation, Injection Sites, and Dosing
It is essential for the body’s antibody levels to be adequate in order to combat viruses, bacteria, or other foreign bodies. Gammagard liquid contains the antibody immunoglobulin G (IgG), which aids in the fight against infections.
Can IVIG help?
Free IVIG Treatment Info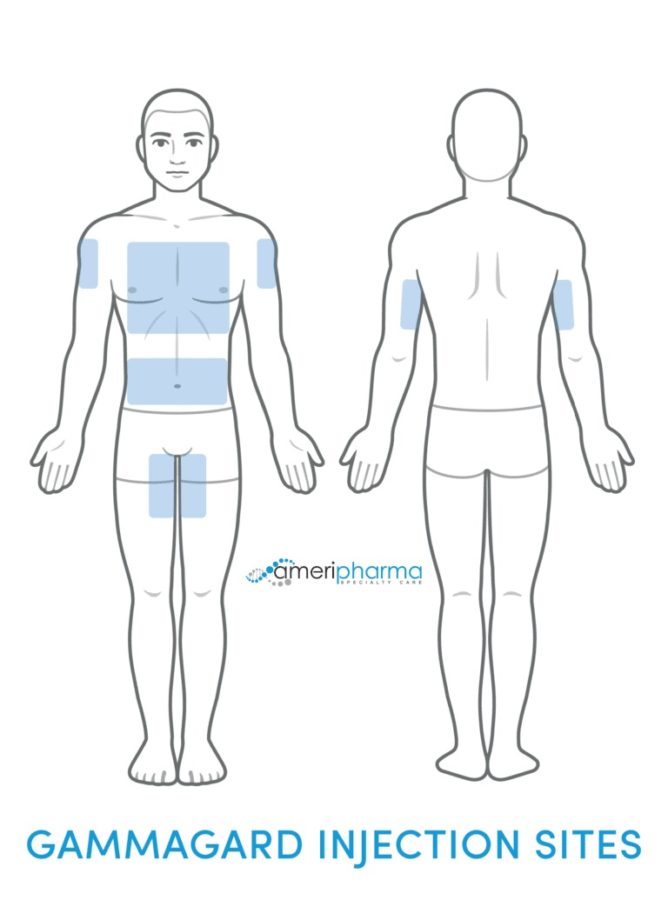
Gammagard IV
- Administered directly into the vein of the patient’s arm (intravenously) by a trained medical professional.
- Frequency: once every 3 to 4 weeks.
Generally administered under the setting of a hospital, clinic, or at a patient’s residence.
Gammagard SubQ
- Needles are placed into the subcutaneous tissue (fatty tissue under the skin).
- Usually in the abdomen, thigh, or upper arm areas.
- Needles may be placed in multiple sites (up to 8 different sites).
- Patients usually receive therapy once a week.
- After receiving training from a medical professional, it is possible for the patient to self-administer their doses independently.
- This therapy is generally administered at home if a patient or caregiver is already familiar with proper administration and has received training.
Get Your At-Home Gammagard IVIG Infusion Now!
Dosing
IV (Intravenous) Administration for PI
Intravenous doses range from 300 – 600 mg/kg every 3 to 4 weeks. The doctor will adjust the dose of Gammagard based on the patient’s clinical response.
Initial infusion rate:
Initiate the rate at 0.5 ml/kg/hr (0.8 mg/kg/min) for 30 minutes. The rate can then be increased based on the tolerability of the patient.
Maintenance infusion rate:
If the infusion is well tolerated, the rate can be increased every 30 minutes up to 5 ml/kg/hr (8 mg/kg/min).
IV (Intravenous) Administration for MMN
Dose:
Intravenous doses range from 0.5 to 2.4 grams/kg/month. Your doctor will adjust the dose based on the patient’s clinical response.
Initial infusion rate:
Initiate the rate at 0.5ml/kg/hr (0.8 mg/kg/min).
Maintenance infusion rate:
If tolerated, the infusion rate can be increased to 5.4 ml/kg/hr (9 mg/kg/min), every 30 minutes.
SubQ Administration for PI
Dose:
The initial dose is calculated at 1.37 times the number of previous intravenous doses, divided by the number of weeks between intravenous doses. The maintenance dose of Gammagard differs based on the patient’s clinical response.
Initial infusion rate:
- If the patient weighs more than 40 kg: initiate the rate at 30 ml/site at 20 ml/hr/site.
- If the patient weighs under 40 kg: initiate the rate at 20 ml/site at 15 ml/hr/site.
Maintenance infusion rate:
- If the patient weighs more than 40 kg: initiate the rate at 30 ml/site at 20 to 30 ml/hr/site.
- If the patient weighs under 40 kg: initiate the rate at 20 ml/site at 15 to 20 ml/hr/site.
Ask About IVIG Home Infusion
Gammagard Uses
A patient is more susceptible to viruses and bacteria when their immune system is compromised. Gammagard IVIG liquid replenishes the antibody levels in individuals to allow them to have an immune system that can overcome these susceptibilities.
In the United States, approximately 250,000 people have been diagnosed with primary immunodeficiency (PI). Fortunately, the liquid has a variety of critical applications, which we will discuss in more detail below.
Sustaining Immunity Against Infection
In one study, Gammagard J code was administered every 3 to 4 weeks for a year to 61 patients with primary immunodeficiency. The dosage used was 300 – 600 mg/kg.
The study concluded that no bacterial infections occurred as a result of this treatment. In addition, out of the 61 patients, none required hospitalization for a primary infection other than a urinary tract infection, gastroenteritis, or ear infection.
According to the same study, the medication’s side effects were tolerable. The most frequently reported adverse effect was a mild headache, which occurred 5.2% of the time.
Treatment for Primary Immunodeficiency
PI is an insufficient immune response. It is inherited and can affect people of all ages and genders. Immunotherapy is required to maintain a PI patient’s health, and Gammagard is one of the drugs for this condition.
Management of MMN
Immune globulins are used in adult patients with multifocal motor neuropathy as a maintenance therapy to improve muscle strength and disability.
Gammagard Cost
This medication has a reasonable price considering all its health benefits. The cost of Gammagard IVIG liquid may vary depending on the pharmacy or clinic a patient visits. However, the following is a rough estimate of the cost of this medication:
| Quantity | Price Per unit | Price |
|---|---|---|
| 10 milliliters | $16.53 | $165.33 |
| 25 milliliters | $15.96 | $399.10 |
| 50 milliliters | $15.77 | $788.69 |
| 100 milliliters | $15.68 | $1,567.88 |
| 200 milliliters | $15.63 | $3,126.26 |
| 300 milliliters | $15.62 | $4,684.64 |
Other IVIG brands include Gamunex-C and Privigen. Privigen is less expensive, costing approximately $16 per 100 ml. Additionally, the starting dose of Privigen is only 50 ml. Gammagard liquid and Gamunex have been shown to have a similar price range.
Copay Assistance
Copay Assistance is available to patients prescribed this drug to treat PI or MMN. Takeda offers an assistance program, which patients can enroll in independently or through a medical provider.
Get IVIG Copay Assistance
Speak to a SpecialistImmune Globulin Shortage
There has been a global shortage of immune globulin beginning in 2019. This shortage occurred due to decreased human plasma donations, particularly during the COVID-19 pandemic.
Fortunately, the FDA has approved Gammagard IVIG liquids and extended their shelf life, thus reducing the chance it will be in short supply.
Tips While on Gammagard Treatment
Follow these tips when receiving treatment outside your home:
- Bring a book, a movie, or other relaxing material to keep you occupied during the infusion. Infusions can last between 2 to 6 hours, depending on the volume of your dose.
- Relax and sit comfortably.
- Inform the nurse if you feel uneasy at any point during the infusion.
- Inform your doctor of any adverse reactions you experience.
- Record your infusion in your logbook.
If a patient is administering the Gammagard medication at home, it is crucial to:
- Visually inspect the product upon receipt for particulate matter and discoloration before administration. This medication is a colorless or pale yellow solution that is clear or slightly opalescent. Do not use the liquid if it is cloudy, turbid, or contains particulates.
- Keep vials refrigerated until use.
- Use vials immediately after opening since the vials are only for single use. Discard partially used vials.
- Allow the refrigerated product to reach room temperature before use.
- Avoid microwaving, shaking, or combining the medication with other products.
FAQs
What is Gammagard Used to Treat?
Doctors use the Gammagard IVIG solution to treat immunodeficiency, autoimmune diseases, multifocal motor neuropathy, and Alzheimer’s disease. In addition, the liquid can treat patients two years and older with primary immunodeficiency.
Is It an Immunosuppressant?
This solution is classified as an immunosuppressant because doctors prescribe it to treat autoimmune conditions.
What Antibodies are in the liquid?
Gammagard contains immunoglobulin G (IgG). These antibodies are isolated and synthesized from the plasma of healthy individuals.
When Do You Use It?
The medication has a multitude of applications and benefits. It may be used in the following situations, depending on the treatment prescribed by a physician:
- Patients with primary immunodeficiency (PI) or multifocal motor neuropathy (MMN)
- When there is a need to prevent bacterial infections in patients with hypogammaglobulinemia (low levels of immune globulin)
- Patients having recurrent bacterial infections associated with B-cell chronic lymphocytic leukemia