Tysabri, also known by its generic name “natalizumab,” is a prescription medication indicated for the treatment of Crohn’s disease and relapsing forms of multiple sclerosis (MS) in adults.
Speak to a Specialist
About Copay AssistanceIn 2004, the FDA authorized its use as the first monoclonal antibody for the treatment of active relapsing-remitting MS (a debilitating disease of the brain and spinal cord). Tysabri is a medication that belongs to the class of monoclonal antibodies.
Tysabri comes in liquid form and is available through a special restricted program called TOUCH. This medication can only be obtained through a prescription.
What Is Tysabri Used To Treat?
Tysabri is used as monotherapy (not in combination with other disease-modifying therapies) to treat the following conditions:
Relapsing Forms of Multiple Sclerosis (MS)
Multiple sclerosis is a chronic autoimmune and debilitating neurological condition where a patient’s immune system mistakenly attacks the myelin sheath (a protective covering) of nerve cells in the brain and spinal cord. This immune attack results in inflammation due to nerve damage, leading to movement disability, blurry vision, slurred speech, chronic pain, and fatigue.
Multiple sclerosis (MS) symptoms typically fluctuate in people with relapsing MS. MS Patients experience new lesions or flare-ups in the existing symptoms for a few days or weeks, followed by the recovery phases, where symptoms may ease or disappear temporarily.
Tysabri is generally recommended as monotherapy to treat individuals with relapsing forms of multiple sclerosis in order to prevent the onset of physical impairment and lessen the frequency of clinical exacerbations. Patients who have not responded well to or cannot handle an alternative MS medication are typically advised to use Tysabri.
Tysabri is designed to help people with relapsing forms of MS experience fewer flare-ups and postpone or avoid physical disability.
Crohn’s Disease (CD)
An inflammatory disorder called Crohn’s disease causes inflammation in the gastrointestinal tract or any of its segments, most frequently the small or large intestine. Although the precise etiology of CD is unknown, it is thought that the condition is brought on by an autoimmune reaction, in which your body’s immune system attacks healthy cells.
The symptoms of CD are bloody stool, diarrhea, fever, fatigue, weight loss, belly cramps, and frequent bowel movements.
Tysabri is generally recommended for patients with moderate to severe CD and has shown an inadequate response to CD therapies such as tumor necrosis factor (TFN) blockers.
Tysabri is used to lessen signs and symptoms in CD patients.
How Does Tysabri Work?
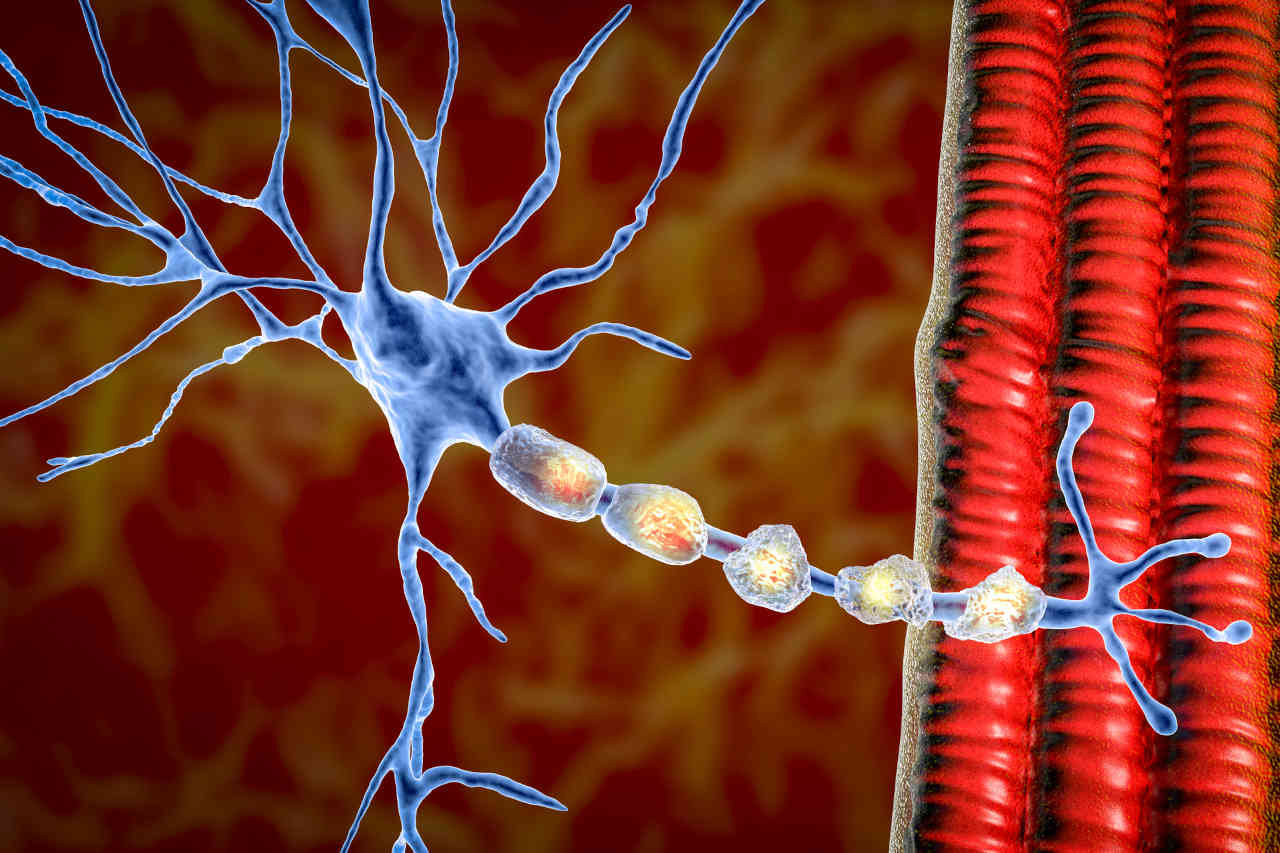
Tysabri contains an active component, “natalizumab,” a type of monoclonal antibody that recognizes and binds to the specific receptor proteins called integrins (with α4β1 and α4β7 subunits) on white blood cells (WBC).
Our immune system is normally composed of white blood cells (WBC), which are responsible for eliminating foreign intruders. In contrast, these cells damage the healthy cells in MS and CD, which results in inflammation.
In MS, natalizumab (the active drug) binds to α4β1 integrin surface proteins of WBC and prevents the white blood cells from crossing the blood-brain barrier (a barrier that protects the brain and spinal cord from potential toxins and hyperactive immune cells). As a result of natalizumab binding to the α4β1 integrins, it reduces nerve damage and inflammation in the brain and spinal tissues.
In the case of CD, the natalizumab binds to α4β7 integrins surface proteins of WBC and prevents the WBC from causing inflammation in the gastrointestinal tract.
Tysabri helps to reduce the symptoms of multiple sclerosis and Crohn’s disease.
Treatment Info
Get Tysabri Prior AuthorizationTysabri Dosage Form and Strength
Tysabri comes in a sterile, concentrated liquid solution for injection in a single-use vial. Tysabri is only available in one strength: 300 mg/15 ml (20 mg/ml).
Since Tysabri comes in concentrated form, it must be diluted before use.
Tysabri dilution procedure:
- Remove the 15 ml Tysabri concentrate (300 mg) from the vial with a sterile syringe and inject it into 100 ml of 0.9% sodium chloride injection.
- Mix the injection content by gently inverting it.
- The final dosage will be 2.6 mg/ml.
- Use the Tysabri dilution within 8 hours.
Tysabri Dosing Information
For MS and CD patients, the recommended Tysabri dosage is a 300 mg IV infusion given over an hour, once every four weeks (one dose per month).
Infusions of Tysabri should be stopped if a CD patient does not see therapeutic results during the first 12 weeks of therapy.
How Is Tysabri Given?
Tysabri is only given intravenously (through veins) for one hour once every four weeks. The patients should be monitored during or after 1-hour IV infusions since infusion can trigger allergic reactions.
If you have missed your infusion appointment, consult your healthcare provider immediately to receive the dose.
Is Tysabri a Form of Chemotherapy?
No, Tysabri is not a chemotherapy but an immunosuppressant drug that works differently than chemotherapy drugs. Unlike chemotherapy which stops cancer growth, Tysabri, as an immunosuppressant, suppresses your immune system.
This suppression helps to manage the symptoms of MS and Crohn’s disease. But on the other hand, immune suppression also raises the risk of infection.
Interaction With Other Drugs
Tysabri should not be used in combination with other immunosuppressant drugs and TNF-blockers as it may increase the risk of developing progressive multifocal leukoencephalopathy (PML).
- Immunosuppressants: Azathioprine, cyclosporine, and methotrexate.
- TNF-blockers: Infliximab, adalimumab, and golimumab.
Possible Side Effects of Tysabri

The common side effects of Tysabri that a patient may experience during or after IV infusion includes:
- Headaches
- Dizziness and nausea
- Fatigue
- Joint pain
- Nasopharyngitis (nose and throat inflammation)
- Mild infections such as urinary tract, vaginal, respiratory, and stomach infections
- Mild allergic reaction
- Diarrhea
Tysabri also has severe and potentially fatal side effects requiring immediate medical attention. Some of the serious side effects that are reported in patients include:
- Progressive multifocal leukoencephalopathy (PML), a rare brain infection that causes severe disability or even death
- Herpes infection that affects the brain or eyes
- Liver damage
- Low platelets and RBC counts
Speak to a Specialist
Precautions
Before receiving Tysabri IV infusions, you must consult your healthcare provider if you:
- Are under age 18, as it is not known whether Tysabri is safe and works in underage patients.
- Are pregnant or plan to become pregnant, as this drug may harm your unborn baby and cause low platelets and RBC counts (anemia) in newborns.
- Are breastfeeding or planning to breastfeed. Since the data on Tysabri secretion in breast milk is limited, avoiding taking Tysabri infusion in such cases is recommended.
- Are taking other immunosuppressants or corticosteroids, as Tysabri use in combination with TNF blockers or immunosuppressant drugs increases the risk of PML infection.
- Have or have had other medical conditions that weaken your immune system, such as HIV, AIDS, leukemia, and organ transplants.
- Have an allergy to Tysabri components.
Cost
One single-dose vial of 300 mg is expected to cost about $8,282. The price of Tysabri may vary depending on the pharmacy you visit and your insurance plan.
REFERENCES:
- US Food and Drug Administration (FDA). Tysabri Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf
- Selewski, D., Shah, G., Segal, B., Rajdev, P., & Mukherji, S. (2010). Natalizumab (Tysabri). American Journal of Neuroradiology, 31(9), 1588–1590. https://doi.org/10.3174/ajnr.a2226
- Johnson KP. Natalizumab (Tysabri) treatment for relapsing multiple sclerosis. Neurologist. 2007 Jul;13(4):182-7. doi: 10.1097/01.nrl.0000263760.53418.5b. PMID: 17622909.
- European Medical Agency (EMA). Tysabri prescribing information. https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf
- TYSABRI® (natalizumab) | Official Patient Website. (n.d.). https://www.tysabri.com/
- Multiple sclerosis – Symptoms and causes. (2022, January 7). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/symptoms-causes/syc-20350269
- Crohn’s disease – Symptoms and causes. (2022b, August 6). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/crohns-disease/symptoms-causes/syc-2035330