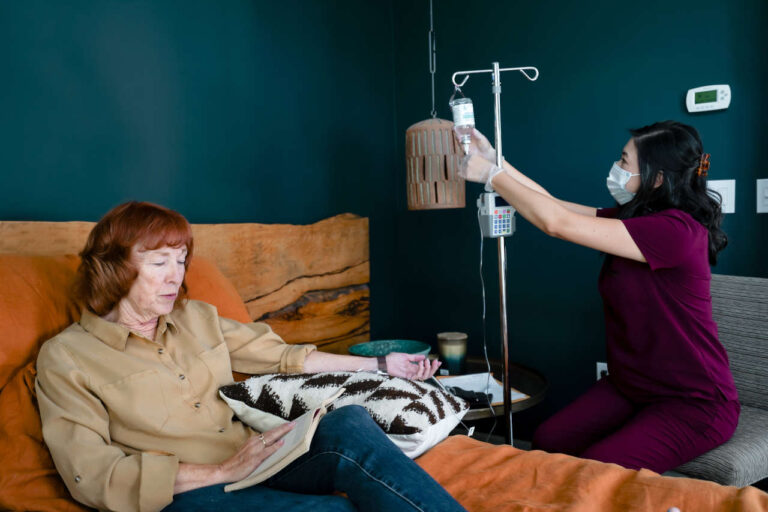
IVIG for hematologic malignancy-associated secondary immunosuppression may be an option when patients have severe or recurrent infections. Discover how IVIG is administered and find answers to the most common questions.
Talk With a Copay Assistance Specialist
Highlights
- Hematologic malignancy-associated secondary immunosuppression means a weakened immune system caused by hematologic malignancies or their treatments.
- IVIG is widely used to prevent recurrent and severe infections in patients with hematologic malignancies.
- A patient may switch from IVIG to SCIG in certain situations.
What Is Hematologic Malignancy-Associated Secondary Immunosuppression?
Let’s first break down the term “hematologic malignancy-associated secondary immunosuppression.”
Hematologic Malignancy
These cancers originate in the bone marrow or the cells of the immune system. The three main groups of hematologic malignancy are:
- Leukemia: Leukemia occurs when the bone marrow produces abnormally high amounts of white blood cells.
- Lymphoma: Lymphoma originates in the part of the immune system called the lymphatic system. Lymphoma affects white blood cells known as lymphocytes.
- Multiple myeloma: This is a rare cancer that causes an overgrowth of specialized white blood cells called plasma cells.
Secondary Immunosuppression
Secondary immunosuppression refers to a weakened immune system due to:
- Disorders of blood or bone marrow
- Medications
- Cancer treatments
- Certain cancers
- Malnutrition
- Infectious diseases
Hematologic malignancy-associated secondary immunosuppression occurs when hematologic malignancies or their treatments cause a weakened immune system. A weakened immune system puts patients at a higher risk of getting recurrent or severe infections.
IVIG (intravenous immunoglobulin) is one of the treatment options for hematologic malignancy-associated immunosuppression. Other management options include:
- Preventive antibiotic treatment and vaccination
- Reduced immunosuppression
- Treatment of the underlying condition
Do You Qualify for $0 Copay?
Talk to a Specialist to Find OutIVIG for Hematologic Malignancy-Associated Secondary Immunosuppression: What We Know So Far

IVIG is widely used to help reduce the risk of severe infections in patients with hematologic malignancies. In these patients, IVIG can prevent recurrent and severe infections [1]. A healthcare provider may consider IVIG for hematologic malignancy-associated secondary immunosuppression when [2,3]:
- Patients have severe or recurrent infections
- Antibiotics are ineffective
- IgG level is less than 4g/l
- Patient is receiving CAR-T Cell therapy
Further Reading: IVIG for CAR-T Therapy-Related Hypogammaglobulinemia: Uses, Benefits, Dosage, and More
However, the use of IVIG for hematologic malignancy-associated secondary immunosuppression is still not clear because:
- The benefits of IVIG for hematologic malignancy-associated secondary immunosuppression come from small clinical trials; robust evidence from large controlled trials is lacking.
- There are no specific clinical treatment guidelines on the use of IVIG in this patient population.
Due to cost and limited supply of IVIG, it is typically recommended for therapy to be individualized based on the patient’s condition and type of cancer therapy.
Treatment Considerations and Dosing
Administering IVIG for hematologic malignancy-associated secondary immunosuppression involves several steps.
First, a healthcare provider will measure the IgG levels before or during cancer treatment. Second, they will determine when to start IVIG therapy, which typically relies on the IgG levels. Lastly, they will determine the dose based on the body weight.
Step 1: Measuring IgG Levels
According to the European expert consensus, in patients with hematologic malignancies [4]:
- Measuring IgG levels before starting cancer treatment can help estimate the patient’s risk of developing infections.
- A healthcare provider may also measure Ig levels during routine visits if the patient has already started cancer treatment.
For patients younger than 21 years, a healthcare provider will interpret the IgG levels based on what’s considered normal for their specific age.
Get Financial Assistance
Step 2: Starting IVIG Therapy
The ideal candidates for IVIG for hematologic malignancy-associated secondary immunosuppression are those with:
- IgG levels less than 4 g/l who have received antimicrobial therapy. IVIG should be started during or after a single severe infection or recurrent infection.
- IgG levels less than 4 g/l who have severe or recurrent infections even after they have received antimicrobial therapy.
While not all experts agree, patients with IgG levels between 4 g/l and 6 g/l who have severe or recurrent infections even after they have received antimicrobial therapy may also receive IVIG treatment.
Step 3: IVIG Dosing
IVIG dose depends on body weight. If the patient is obese, dosage adjustment according to the ideal body weight specific to the patient is necessary.
The dosing regimen of IVIG for hematologic malignancy-associated secondary immunosuppression is as follows [3]:
- The starting dose of IVIG is 0.4 g/kg body weight every 3 to 4 weeks.
- The maintenance dose of IVIG is 0.4 to 0.8 g/kg body weight every 3 to 4 weeks.
IVIG for Hematologic Malignancy-Associated Secondary Immunosuppression: Special Considerations
- When starting IVIG therapy, a healthcare provider may consider stopping antimicrobial treatment if the infection is under control.
- If the patient doesn’t have an infection for a significant period of time (typically 6 months), a healthcare provider may stop IVIG therapy.
- Patients who no longer use IVIG should get their IgG levels tested during routine visits.
- Should severe or persistent infections recur in patients who no longer use IVIG, IVIG treatment can be restarted.
- IVIG typically causes mild infusion-related reactions, such as headache, nausea, and muscle aches.
- Rare but severe side effects include severe allergic reactions, kidney failure, and rupture of red blood cells. A healthcare provider will closely monitor patients during IVIG therapy if they have risk factors for these serious side effects.
IVIG for Hematologic Malignancy-Associated Secondary Immunosuppression: Can A Patient Switch to Subcutaneous Immune Globulin (SCIG)?
A patient can switch to SCIG after discussing the potential benefits and risks with their healthcare provider. The major benefit is that they can self-administer SCIG at home after proper training. SCIG typically also has fewer side effects than IVIG. They may choose to switch to SCIG if:
- They are ready to take charge of their health.
- Other forms of treatment fail to provide adequate IgG levels.
- Specific immunoglobulin formulations are unavailable.
Get Copay Assistance Now
Frequently Asked Questions
What hematologic malignancy is associated with secondary immunosuppression?
Chronic lymphocytic leukemia and multiple myeloma are often associated with secondary immunosuppression.
Which type of virus has been associated with hematologic malignancies?
Viruses associated with hematologic malignancies include HIV, Epstein–Barr virus (EBV), and Kaposi sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8).
What is the most common cause of secondary immunodeficiency?
The most common causes of secondary immunodeficiency are:
- Blood or bone marrow disorders
- Cancer treatments
- Certain cancers
REFERENCES:
- Goede, Jeroen S., et al. “Rational Use of Immunoglobulins (IVIgs and SCIgs) in Secondary Antibody Deficiencies.” Swiss Medical Weekly, vol. 154, no. 9, Sept. 2024, p. 3559, doi:10.57187/s.3559.
- Immunoglobulin Therapy for Secondary Immunodeficiencies Associated With Haematological Malignancies » SID. www.secondaryimmunodeficiency.com/immunoglobulin-therapy.
- Dimou, Maria, et al. “Management of Secondary Immunodeficiency in Hematological Malignancies: A Delphi Consensus From the Middle East.” Frontiers in Hematology, vol. 3, Mar. 2024, doi:10.3389/frhem.2024.1347708.
- Jolles, Stephen, et al. “Treating Secondary Antibody Deficiency in Patients With Haematological Malignancy: European Expert Consensus.” European Journal of Haematology, vol. 106, no. 4, Jan. 2021, pp. 439–49, doi:10.1111/ejh.13580.