Gammaplex is a highly purified human intravenous immunoglobulin G (IVIG). It helps prevent infection in primary immunodeficiency diseases (PIDDs) and bleeding in immune thrombocytopenic purpura (ITP). It is available as an intravenous liquid solution at different concentrations.
What Is Gammaplex?
Gammaplex infusions use plasma from paid, healthy US subjects. The manufacturers use plasmas of approximately 3,000 to 10,000 donors to obtain human immunoglobulin derivatives [1].
This infusion is available in two concentrations: 5% and 10% liquid high-purity formulations (>95% for 5% and >98% for 10%) [2].
These formulations differ in their stabilizer. For example, the 10% solution is stabilized with glycine and doesn’t contain carbohydrate-reducing stabilizers. Meanwhile, the 5% solution is stabilized with sorbitol.
Get IVIG Copay Assistance
Speak to a SpecialistCharacteristics
The distribution of IgG subclasses in Gammaplex products reflects that of normal plasma [3].
In addition, the IgA content is low, reducing the risk of hypersensitivity reactions in IgA-deficient patients.
During treatment, doctors strictly control the anti-D and anti-A/anti-B hemagglutinin contents to reduce the risk of hemolytic reactions.
There is very low or undetectable procoagulant activity, as shown by testing multiple batches from the starting plasma pool to the final product.
Overview of IVIG Products
Different immune globulin products licensed in the United States are available, such as Gammaplex, Gamunex, Flebogamma, Octagam, Bivigam, Gammaked, and Privigen.
These products differ in many aspects, such as:
- The manufacturing process
- The formulation (liquid versus dry powder)
- Concentration
- The stabilizer used
- Composition
- Viral inactivation
- pH
- Volume load
- Osmolality
- Sodium content
- Method of administration
Gamunex is given either intravenously (abbreviated as IVIG) or subcutaneously (abbreviated as SCIG), while Gammaplex is administered only as an intravenous immunoglobulin IVIG infusion.
IVIG products are formulated as either liquid (such as Gammaplex) or dry powder (lyophilized, like Gammagard S/D). For the manufacturing process, immune globulin products differ in the isolation and purification of IgG, and the antibody fraction.
All these factors affect the final product’s shelf life, efficacy, and tolerability. As a result, certain products may be selected for a specific group of patients.
For example, patients with cardiovascular risk factors or small infants should be given products with low sodium content and osmolality [4]. Diabetic patients should not receive IVIG products that contain glucose. Those with preexisting renal conditions shouldn’t use products that contain sucrose.
What Is Gammaplex Used For?
Gammaplex is an FDA-approved product for managing primary humoral immunodeficiency diseases (PIDDs) in adults and pediatric patients 2 years old and above. In addition, Gammaplex infusions help prevent bleeding in idiopathic thrombocytopenic purpura (ITP).
Immunodeficiency disorders result from the absence or dysfunction of a part of the immune system. Immunodeficiency disorders can have two categories: primary and secondary.
The principal clinical manifestation of primary humoral immunodeficiency (PI) diseases is an increased risk of infection. Almost all patients suffered serious or unusual infections before receiving Gammaplex treatment. A large population reported suffering from permanent functional impairment [5].
Consult an IVIG Specialist
The Best IVIG Home Infusion | Get IVIG Treatment AssistancePrimary Humoral Immunodeficiency (PI)
Primary immunodeficiency is divided into several types according to their dysfunctional element:
- T-cell deficiency
- B-cell deficiency
- Both T-cell and B-cell deficiency
- Complement deficiency
- Phagocyte deficiency
- Immunoglobulin A deficiency [6]
Primary Humoral Immunodeficiency often has an underlying genetic cause.
For example, in primary immunodeficiency, B-cell deficiency is an X-linked disorder. It is called X-linked agammaglobulinemia (Bruton disease) [7].
Babies with X-linked agammaglobulinemia suffer from recurrent bacterial infections, including otitis media, bronchitis, septicemia, pneumonia, arthritis, and giardia lamblia, which causes intestinal malabsorption.
Administering intravenous immune globulins such as Gammaplex infusions can help patients with X-linked agammaglobulinemia. Gammaplex can keep them alive by replacing low levels of immunoglobulins.
Another subcategory of primary humoral immunodeficiency is T-cell Immunodeficiencies, including Hyper-IgM syndrome. Doctors recommend immunoglobulin therapy for this syndrome.
This treatment is also helpful for congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
Chronic Immune Thrombocytopenic Purpura (ITP)
Immune thrombocytopenic purpura (ITP) is isolated thrombocytopenia (platelet count <100,000/microL) with normal white blood cells and normal hemoglobin. It occurs in the setting of a generalized purpuric rash as defined by the American Society of Hematology [8].
ITP is also known as idiopathic thrombocytopenic purpura or immune thrombocytopenic purpura.
According to lab tests, the white blood count, hemoglobin concentration, red cell indices, and differential white blood cells are usually normal. The only abnormality before Gammaplex treatment is a platelet count of less than 100,000/microL [9].
The etiology of ITP includes the development of autoantibodies against platelet membrane proteins, leading to thrombocytopenia and an elevated risk of bleeding. ITP is an autoantibody-mediated disorder, usually mediated by immunoglobulin G.
The 2019 American Society of Hematology guidelines recommends using intravenous immunoglobulin (IVIG) for patients who cannot use corticosteroids.
Researchers believe immune globulin intravenous human liquids such as Gammaplex raise platelet counts for patients with chronic immune thrombocytopenic purpura.
Can IVIG help?
Free IVIG Treatment Info | Difficulty In Diagnosing?Gammaplex Mechanism of Action
In primary humoral immunodeficiency, Gammaplex acts as an IgG replacement therapy by replacing the low levels of opsonic and neutralizing IgG antibodies, thus boosting the immune system against pathogens and their toxins.
It may downregulate antiplatelet antibody production and block Fc receptors in chronic ITP, but the exact mechanism is still unclear [10].
Dosing
The dosage of Gammaplex infusions varies according to the indication and the concentration administered.
Treatment of Primary Humoral Immunodeficiency
For treating primary humoral immunodeficiency disease, the frequency and the dose of immunoglobulin may vary from patient to patient. This difference is because the half-life of IgG varies among patients.
The proper amount can be adjusted according to the patient’s clinical response.
However, according to the Gammaplex package insert, Gammaplex 5% (100 ml) is given intravenously at a dose of 300 to 800 mg/kg (6-16 mL/kg) every 3 to 4 weeks for managing PI.
Gammaplex 10% (50 ml) is administered intravenously at similar doses. But this higher concentration allows a 34% reduction in infusion time without compromising safety and tolerability.
Treatment of Chronic Immune Thrombocytopenic Purpura
The recommended dose of the 5% solution for patients with ITP is 1 g/kg (20 mL/kg) on two consecutive days, providing a total of 2 g/kg.
For patients at increased risk of thrombosis, hemolysis, acute kidney injury, or volume overload, there should be a careful evaluation of the risks and benefits of prescribing this regimen.
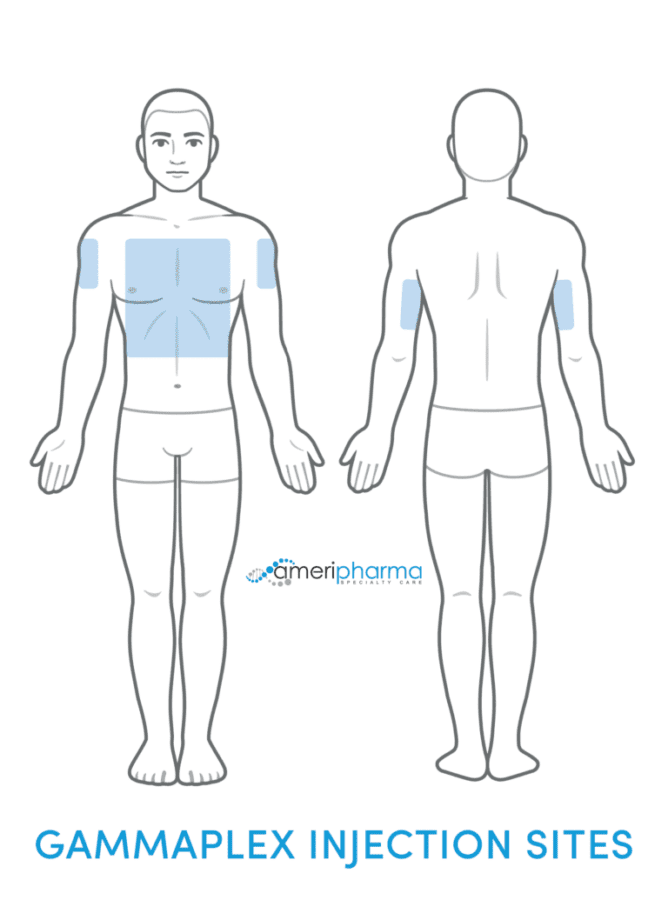
Infusion Rates
What are the infusion rates for managing PI using Gammaplex 5%?
Initially, the infusion rate should be 0.5 mg/kg/min for 15 minutes and increased gradually every 15 minutes to 4 mg/kg/min as a maintenance infusion rate.
What are the infusion rates for managing ITP using the 5% solution?
Initially, the infusion rate should be 0.5 mg/kg/min for 15 minutes and increased gradually every 15 minutes to 4 mg/kg/min as a maintenance infusion rate until the administration of the total dose.
What are the infusion rates for treating PI using Gammaplex 10%?
For managing PI using the 10% solution, the starting infusion rate is set at 0.5 mg/kg/min for 15 minutes and increased gradually every 15 minutes to 8 mg/kg/min.
What are the infusion rates for treating ITP using the 10% solution?
For managing ITP, the initial gammaplex infusion rate is set at 0.5 mg/kg/min (0.005 mL/kg/min) for 15 minutes and increased gradually every 15 minutes to 8 mg/kg/min (0.08 mL/kg/min).
Consult an IVIG Specialist
The Best IVIG Home Infusion | Get IVIG Treatment AssistanceGammaplex Administration
Before administering the drug, the patient should drink plenty of water to ensure adequate hydration.
Doctors should monitor the patient’s vital signs throughout the infusion session.
Patients that show any signs of adverse reactions should receive lower infusion rates. If the adverse reaction subsides, the infusion should be resumed but at a lower rate that is comfortable for the patient.
If the adverse reaction persists, the infusion should stop altogether.
It is vital to ensure that patients with preexisting renal insufficiency are not volume depleted. Patients predisposed to renal dysfunction, thrombotic events, or volume overload should receive Gammaplex infusion at the minimum practicable rate.
Gammaplex Adverse Reactions
IVIG may have adverse reactions.
According to some reports, as many as 21% of patients discontinued their immunoglobulin therapy due to adverse reactions (low tolerability) or concerns about product safety.
Generally speaking, most patients tolerate IVIGs well. Most adverse reactions are mild, transient, and reversible by slowing or temporarily stopping the infusion.
More specifically, most people tolerate Gammaplex IV well, as reflected by the low rate of treatment discontinuations due to side effects.
Similarly, patients with ITP completed 90 of 94 infusions at the maximum recommended rate, indicating that Gammaplex infusions were well tolerated even at high doses.
Adverse Reactions in Subjects With PI
According to the package insert, the most common adverse reactions reported in primary immune deficiency clinical trials were:
- Headache
- Migraines
- Fever
Adverse Reactions in Subjects With ITP
The most common adverse reactions reported in immune thrombocytopenic purpura clinical trials were:
- Headache
- Vomiting
- Fever
- Nausea
- Joint pain
- Dehydration
The frequency of Gammaplex adverse reactions tends to be directly proportional to the rate and dose of infusion. Fortunately, most of these side effects tend to be self-limited, mild, and transient. Usually, these adverse reactions occur during or within 6 hours of infusion, especially after the first infusion session.
Adverse reactions can also be associated with rapid infusion rates, higher doses, switching to a new product, waiting for longer intervals between infusion sessions, and intercurrent infections.
Managing Gammaplex Side Effects

Some simple methods can help prevent side effects from Gammaplex infusions.
For example, drinking plenty of water before or after the infusion can often be enough to minimize the occurrence of adverse reactions [11]. If side effects still occur, doctors can use simple pharmacological approaches, such as administering 500 mg of oral paracetamol or 500 mg of intravenous acetylsalicylic acid.
Another option can be administering non-steroidal anti-inflammatory drugs (e.g., ibuprofen 10 mg/kg), second-generation antihistamines, or corticosteroids.
However, the patient and health care provider should know that sometimes threatening adverse reactions may occur. Adverse reactions of Gammaplex include renal dysfunction/failure, thrombotic events, hypersensitivity, hyperproteinemia, increased serum viscosity, and hyponatremia.
Learn More About Side Effects
Renal Dysfunction/Failure Adverse Reactions
The risk of renal dysfunction associated with IVIG occurs due to the stabilizer utilized.
Renal dysfunction related to IVIGs stabilized with sucrose includes acute renal failure, osmotic nephrosis, and renal insufficiency. Reports of renal dysfunction with sucrose-free IVIGs are relatively rare.
Patients at risk of developing acute renal failure can belong to two distinct populations:
- Patients with underlying conditions
- Patients at an increased risk for developing renal insufficiencies, including those with renal insufficiency, diabetes mellitus, advanced age (>65 years old), volume depletion (dehydration or hypervolemia), sepsis, or paraproteinemia. Patients receiving known nephrotoxic drugs are also at risk.
Since Gammaplex 10% is stabilized with glycine and the 5% solution is stabilized with sorbitol, both products are less prone to cause renal dysfunction or acute renal failure.
Minimizing the Risk of Renal Dysfunction or Acute Renal Failure
Healthcare authorities, including the FDA and clinical experts, recommend several measures to minimize the risk of renal dysfunction or acute renal failure for at-risk patients.
Patients should be hydrated well to ensure that they aren’t hypovolemic before a Gammaplex infusion.
Patients at risk of developing renal dysfunction should receive an infusion at the minimum rate practicable. These patients include those predisposed to acute renal failure.
In addition, patients at risk of developing renal dysfunction should be assessed periodically for their renal function. Patients should discontinue infusions if there is any deterioration in renal function.
Thrombotic Adverse Reactions
IVIG products, including Gammaplex, may increase blood viscosity, possibly impairing blood flow and triggering a cardiovascular or cerebrovascular thromboembolic event.
In one clinical trial, serum viscosity was measured before and immediately after each of three consecutive monthly infusions of IVIG. Serum viscosity increased after IVIG in all 13 patients who participated in the study [12].
Patients at risk of developing thromboembolic events include those:
- Of advanced age
- With prolonged immobilization
- With hypercoagulable conditions
- With a history of venous or arterial thrombosis
- Who use estrogen
- Who have indwelling central vascular catheters
- With hyperviscosity
- With cardiovascular risk factors
However, thromboembolic events from Gammaplex therapy may still occur without these known risk factors.
Minimizing Risk of Thromboembolic Events
Doctors should also assess the patient’s risk for clotting. Patients with uncontrolled blood pressure, high cholesterol levels, and history of stroke or clotting are at a higher risk for developing clots on immunoglobulin therapy.
Patients who are not receiving treatment for these health conditions should get treated before starting their immunoglobulin infusion. Adjustments to the infusion rate and the addition of a hydration infusion can also help to reduce clotting risk.
Patients at risk of thrombosis should receive Gammaplex infusion at the minimum dose and a practicable infusion rate.
Hypersensitivity
Like any other medicinal product, the administration of Gammaplex may cause hypersensitivity reactions.
Hypersensitivity reactions may be due to the trace amounts of IgA in the drug. Patients with known antibodies to IgA are at increased risk of developing hypersensitivity and anaphylactic reactions, so they should be monitored closely.
Patients should discontinue the infusion immediately and seek proper management if a hypersensitivity reaction occurs.
Ask an IVIG Specialist about Side Effects
Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
Hyperproteinemia, high serum viscosity, and hyponatremia are rare adverse reactions that may accompany Gammaplex treatment.
Doctors should assess the patients carefully for signs of hyponatremia and distinguish it clinically from pseudo-hyponatremia.
Pseudohyponatremia may accompany hyperproteinemia, and any management procedure for correcting pseudohyponatremia may further deplete volume and increase the risk of thrombotic events.
Aseptic Meningitis Syndrome
Aseptic Meningitis Syndrome (AMS) may occur in patients on IVIG treatment, especially at high doses (2 g/kg) and rapid infusion rates.
Aseptic meningitis syndrome (AMS) should be suspected if the following signs and symptoms are noticed within several hours to 2 days following IVIG treatment:
- Nuchal rigidity
- Painful eye movements
- Photophobia
- Fever
- Severe headache
- Drowsiness
- Nausea
- Vomiting
Patients experiencing these symptoms from Gammaplex should receive an extensive neurological examination to rule out other causes of meningitis.
Fortunately, discontinuing IVIG treatment usually is enough for the remission of aseptic meningitis syndrome without adverse consequences.
Hemolysis
IVIG, including Gammaplex infusions, may cause hemolysis due to the content of blood group antibodies that can act as hemolysins.
Acute hemolysis may develop into delayed hemolytic anemia, which has been reported upon the use of IVIG therapy. Severe acute hemolysis may also develop into renal dysfunction or disseminated intravascular coagulation.
Some of the main reasons patients may develop hemolysis with the use of Gammaplex are:
- Administration of high doses (i.e., ≥2 g/kg).
- Being other than O-blood type.
Hemolysis can develop in patients with either PI or ITP.
Minimizing the Risk of Hemolysis
Doctors should closely monitor patients at risk of developing hemolysis.
Other measures to minimize the risk of hemolysis should include baseline laboratory testing (such as hemoglobin and hematocrit levels) for patients predisposed to developing hemolysis. Doctors should conduct these tests 36 to 96 hours after infusion.
If hemolysis is suspected, doctors should also conduct confirmatory laboratory tests.
Transfusion-Related Acute Lung Injury (TRALI):
Transfusion-related acute lung injury (TRALI) is one of the adverse reactions that may accompany the transfusion of blood products, including intravenous immune globulin products like Gammaplex.
Transfusion-related acute lung injury is a clinical syndrome. It involves acute, noncardiogenic pulmonary edema that may occur during or after the transfusion session and is associated with hypoxia.
The signs and symptoms of TRALI may include fever, hypotension, and exudative bilateral infiltrates on a chest radiograph.
Transfusion-related acute lung injury is suspected when there are other risk factors for acute lung injury, and symptoms usually appear within 1 to 6 hours following treatment. When transfusion-related acute lung injury is suspected, doctors should perform anti-neutrophil antibody tests for the products and the patient’s serum.
Gammaplex Infusion Costs
The price for Gammaplex intravenous solution 5% is between $890 and $940 for a supply of 100 ml, depending on the pharmacy you visit [9].
The price for this intravenous solution at 10% is between $1,780 and $1,950 for a supply of 100 ml, depending on the pharmacy you visit.
Treatment Info
Get IVIG Prior AuthorizationGammaplex Copay Assistance Programs

Multiple Patient Assistance Programs (PAPs) support low-income, uninsured, or underinsured people. These patients can receive medications for free or at a discounted price.
Pharmaceutical companies sponsor programs such as the Patient Access Network Foundation (PAN), HealthWell Foundation Copay Program, and Bio Products Laboratory (BPL) Reimbursement Support Program.
For instance, in the Bio Products Laboratory (BPL) Reimbursement Support Program, patients can receive up to $2,000 in assistance yearly if they prove they need financial aid.
Gammaplex Manufacturer
The manufacturer of this drug is Bio Products Laboratory.
Is It the Same as Gammagard?
Both are immune globulin products. However, patients can receive Gammagard infusions intravenously or subcutaneously, while they can receive Gammaplex only through an IV.
Another difference between these immune globulin products is their indications for use.
Gammaplex infusion is a replacement therapy for PIDDs and for managing ITP.
Gammagard is also indicated as a replacement therapy for PIDD. But it can also be used as a maintenance therapy to improve muscle strength and disability in adult patients with Multifocal Motor Neuropathy (MMN).
In general, Gammagard costs less. For example, Gammagard 10% (100 ml) would cost nearly $1,600, while a comparable amount of Gammaplex 10% would cost $1,800.
In addition, these products have different manufacturers. The manufacturer of Gammaplex is Bio Products Laboratory. Gammagard’s manufacturer is Baxalta.
Conclusion
Gammaplex is an immunoglobulin product currently licensed in the United States. It is a safe, effective replacement therapy for primary humoral immunodeficiency (PIDD). It also is a management therapy in chronic immune thrombocytopenic purpura (ITP).
Gammaplex infusions are available in two concentrations: 5% and 10%. The higher concentration ensures shorter infusion times.
This product is a well-tolerated medication with mild, self-limited side effects that patients can manage successfully using simple measures. However, more serious adverse reactions are possible, and users should exercise caution.
Gammaplex is not the same as Gammagard. Each is formulated in a particular manner and has different indications.
REFERENCES:
- Gelfand E. W. (2005). Critical decisions in selecting an intravenous immunoglobulin product. Journal of infusion nursing : the official publication of the Infusion Nurses Society, 28(6), 366–374. https://doi.org/10.1097/00129804-200511000-00003
- DailyMed – GAMMAPLEX- human immunoglobulin g solution. Dailymed.nlm.nih.gov. (2021). Retrieved 17 July 2021, from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6c3ce068-59f0-413b-9f93-85fb597d55e5.
- Roberts, P. L., Dolan, T., Paddick, M., Stagg, S., & More, J. E. (2015). Development of an intravenous immunoglobulin with improved safety and functional activity. Biologicals : journal of the International Association of Biological Standardization, 43(2), 123–129. https://doi.org/10.1016/j.biologicals.2014.11.005
- Saeedian, M., & Randhawa, I. (2014). Immunoglobulin replacement therapy: a twenty-year review and current update. International archives of allergy and immunology, 164(2), 151–166. https://doi.org/10.1159/000363445
- Wasserman R. L. (2017). Gammaplex® 5 and 10% in the treatment of primary immunodeficiency and chronic immune thrombocytopenic purpura. Immunotherapy, 9(13), 1071–1088. https://doi.org/10.2217/imt-2017-0071
- Justiz Vaillant AA, Qurie A. Immunodeficiency. [Updated 2021 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500027/
- Hernandez-Trujillo, V. P., Scalchunes, C., Cunningham-Rundles, C., Ochs, H. D., Bonilla, F. A., Paris, K., Yel, L., & Sullivan, K. E. (2014). Autoimmunity and inflammation in X-linked agammaglobulinemia. Journal of clinical immunology, 34(6), 627–632. https://doi.org/10.1007/s10875-014-0056-x
- Rodeghiero, F., Stasi, R., Gernsheimer, T., Michel, M., Provan, D., Arnold, D. M., Bussel, J. B., Cines, D. B., Chong, B. H., Cooper, N., Godeau, B., Lechner, K., Mazzucconi, M. G., McMillan, R., Sanz, M. A., Imbach, P., Blanchette, V., Kühne, T., Ruggeri, M., & George, J. N. (2009). Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood, 113(11), 2386–2393. https://doi.org/10.1182/blood-2008-07-162503
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866. (2020). Blood advances, 4(2), 252. https://doi.org/10.1182/bloodadvances.2019001380
- Jin, F., & Balthasar, J. P. (2005). Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Human immunology, 66(4), 403–410. https://doi.org/10.1016/j.humimm.2005.01.029
- Šutová, I., Chovancová, Z., & Litzman, J. (2019). Adverse effects of immunoglobulin therapy. Nežádoucí účinky imunoglobulinové léčby. Vnitrni lekarstvi, 65(2), 131–132.
- Dalakas M. C. (1994). High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology, 44(2), 223–226. https://doi.org/10.1212/wnl.44.2.223