免疫球蛋白 是由人体产生的蛋白质组成的物质,用于保护和帮助抵抗感染。这些蛋白质也称为抗体。 Gammagard 是其中之一 最常用的免疫球蛋白产品。
免疫球蛋白产品是从数千名捐献者的血液(血浆)中提取的。通过人血浆,可以从捐献血液中分离抗体并用于制造这种药物。武田制药公司生产这种产品,也称为免疫球蛋白,商品名为 Gammagard 液体静脉注射. 它最初用于治疗和控制阿尔茨海默病。
它还已获得FDA批准用于治疗原发性免疫缺陷症。原发性免疫缺陷症是指人体抗体数量不足,导致频繁或反复感染。通过免疫球蛋白疗法,将从健康个体中采集的抗体用于需要补充这些缺失抗体的患者。该疗法已获批用于 两岁及以上 谁是 被诊断患有这种疾病。
2024年1月,美国食品药品监督管理局 (FDA) 批准Gammagard液体制剂用于治疗CIDP成人患者。该批准允许Gammagard用于改善这些患者的神经肌肉功能障碍和损伤。
Gammagard 是 10% IgG溶液(100毫克/毫升),不含蔗糖、添加糖、钠、防腐剂和脯氨酸。因此,该药物非常适合对这些添加剂过敏的人士。
据制造商称,Gammagard IVIG 液体已被证明在多种疗法中安全有效。这主要归功于所有捐献者都经过了潜在的感染筛查。此外,所有人体血浆均仅在 FDA批准 位置。
获得 IVIG 共同支付援助
与专家交谈Gammagard 治疗/疗法

β-淀粉样蛋白(缩写为Aβ)在人体各处合成。当这些蛋白质聚集并在脑内形成斑块时,就会引发神经退行性病变。这一过程会导致记忆力丧失和认知障碍,就像阿尔茨海默病患者所见的那样。免疫球蛋白可以帮助降低 脑内的Aβ毒性.
根据临床研究,患有轻度阿尔茨海默病的患者接受 Gammagard 李quid 患者的血清中 Aβ 抗体水平较高,脑内 Aβ 蛋白聚集水平较低。这使得患者的 MMSE(简易精神状态检查)评分在一年半以上内保持稳定。
另一项研究报告 改善了轻度至中度阿尔茨海默病患者血浆细胞因子水平的变化。MRI扫描显示心脏扩大有所缩小。
虽然这些早期研究表明Gammagard在治疗阿尔茨海默病方面具有潜在益处,但随后进行的更大规模的3期临床试验并未证明其在改善轻度至中度阿尔茨海默病患者的认知或功能方面有效。因此,Gammagard目前尚未获批用于治疗阿尔茨海默病。
美国食品药品监督管理局 (FDA) 已批准 Gammagard IVIG 液体用于治疗免疫缺陷和自身免疫性疾病。这些疾病包括原发性免疫缺陷 (PI) 和 多灶性运动神经病 (MMN)。
当人体免疫系统功能低下,无法产生足够的抗体时,就会发生PI。PI患者需要接受免疫球蛋白治疗以保持健康。
当免疫系统攻击人体神经时,就会发生 MMN。Gammagard 用于维持成年 MMN 患者的肌肉力量。
您的 IVIG 治疗信息
获得 IVIG 事先授权Gammagard 副作用
这种疗法有一些众所周知且可能严重的副作用,包括恶心、呕吐和腹泻。
副作用可能因诊断、给药途径和治疗耐受性而异,但总体而言,许多患者的耐受性良好。以下是接受 PI 和 MMN 治疗的患者常见的副作用。
PI 常见副作用
静脉注射:
- 皮疹或皮肤瘙痒
- 发冷
- 咳嗽
- 突然感到寒冷,伴随体温升高(寒战)
- 发烧
- 哮喘
- 疲劳
- 恶心
- 肌肉疼痛(肌痛)
- 腹泻
- 偏头痛
- 头晕
- 呕吐
- 关节僵硬(关节痛)
- 头痛
- 心脏杂音
- 四肢积液或疼痛
皮下给药:
- 疲劳
- 发烧
- 恶心
- 哮喘
- 耳痛
- 腹泻
- 偏头痛
- 呕吐
- 头痛
- 心率加快
- 上腹部疼痛
- 四肢疼痛
- 输注部位(局部)反应
- 收缩压升高
MMN 常见副作用
常见的副作用包括:
- 恶心
- 头痛
- 肌肉痉挛
- 胸部不适
- 肌肉无力
- 头部或颈部疼痛(口咽痛)
- 四肢疼痛
如果您遇到副作用或有任何疑问,请立即联系医疗专业人员。
如果您出现严重的副作用,请寻求紧急医疗救治。请致电1-800-FDA-1088,或尽快联系您的医生。
该药的 包装说明书 包含有关严重副作用的附加信息 Gammagard 免疫球蛋白 解决方案。
常见的注射部位副作用
- 轻度至中度疼痛
- 肿胀
- 瘙痒
- 瘀伤
- 发红
- 低烧
- 温暖
上述副作用主要在注射部位明显。这些副作用通常会在注射后几小时内消退。但是,如果您出现任何副作用,仍需尽快告知医生。
常见的一般副作用
- 轻微头痛
- 偏头痛
- 注射部位发红或发热
- 疲劳
- 低烧
- 瘙痒或皮疹
- 咳嗽
- 胸痛
- 发冷
- 呼吸困难
- 头晕
- 恶心和呕吐
- 心率加快
- 血压升高
- 上腹部疼痛
- 肌肉痉挛和虚弱
- 咽喉痛
最初几次输液后,这些副作用发生的可能性较小。
副作用 Gammagard MMN 液体注射
以下是Gammagard液体注射剂治疗MMN(多灶性运动神经病)的一些副作用。列出的副作用较为罕见,发生率较低:
- 严重头痛
- 胸痛
- 肌肉痉挛和无力
- 呼吸困难
- 恶心
- 咽喉痛
- 头晕
- 发红
- 发烧
- 耳朵、手和脚疼痛
咨询 Gammagard IVIG 专家关于副作用
严重的副作用
以下列出的是服用期间可能出现的严重且罕见的副作用 Gammagard:
过敏反应
- 麻疹
- 口腔或喉咙肿胀或发紧(喘息)
- 低血压
- 心跳加速
- 呼吸困难
- 头晕并昏厥
肾脏问题
- 肾衰竭导致排尿减少
- 体重增加
- 腿部肿胀(可能是肾衰竭的征兆)
肝脏问题
- 皮肤或眼睛发黄
血栓(血栓形成)
- 腿部和手臂疼痛和肿胀
- 棕色、红色或深色尿液
- 心率过快
心脏和肺部并发症
- 胸痛
- 呼吸困难
- 嘴唇和四肢发青
感染风险
- 发烧超过 100华氏度
无菌性脑膜炎综合征
- 发烧
- 发冷
- 身体疼痛
- 对光敏感
- 食欲不振
- 呕吐
- 疲劳
- 恶心
- 落枕
溶血(红细胞破坏)
- 皮肤异常苍白
- 皮肤或眼睛发黄(黄疸症状)
- 深色尿液
- 发烧
- 弱点
- 头晕
- 困惑
输血相关急性肺损伤
以下症状通常发生在 Gammagard 治疗:
- 呼吸困难或喘息(肺水肿的迹象)
- 皮肤颜色变化、意识模糊、咳嗽、心率加快、呼吸急促、呼吸急促、心率减慢、出汗(以下均为缺氧症状)
- 发烧
- 可传播的感染因子(由于该药物来自人体血浆,因此可能具有传播感染因子的风险)
重要的是要认识到,本节列出的警告或副作用并非都可能发生 Gammagard 免疫球蛋白 解决方案。如果患者出现过敏反应或严重副作用,请立即联系医疗专业人员(致电1-800-FDA-1088,或尽快联系您的医生)。
考虑阅读 Gammagard的包装说明书 如果您想更详细地了解这些常见且严重的副作用。
IVIG 输注不起作用吗?
最佳IVIG家庭输液方案 | IVIG适应症不良反应

不良反应是指药物治疗引起的副作用。以下是使用以下药物可能引起的一些严重不良反应的症状: Gammagard:
- 呼吸困难
- 皮疹
- 肾衰竭
- 心脏和肺部血栓
- 严重头痛和疲劳
- 发烧
- 发冷
- 眼球运动疼痛
- 对光敏感
- 恶心和呕吐
- 睡意
- 深色尿液
- 肌肉肿胀
如果患者出现本节所列的严重不良反应的体征和症状,请联系紧急服务部门。拨打1-800-FDA-1088,或立即通知您的医生。
包装说明书 Gammagard SD 卡可以访问 这里,其中包含有关这些不良反应的更多信息。
注意事项和禁忌症
Gammagard 免疫球蛋白 液体疗法是一种非常有益的药物,尤其适用于免疫缺陷患者。每位患者在开始治疗前都应接受以下彻底筛查。
- 对免疫球蛋白和血液制品过敏
- 选择性或严重免疫球蛋白A(IgA)缺乏症
Gammagard 含疫苗的液体
Gammagard 疗法可能会损害免疫反应并降低 活疫苗 例如麻腮风(MMR)或水痘。如果您目前正在接受免疫球蛋白治疗,请告知您的免疫医生。
Gammagard 孕期液体
Gammagard 疗法被归类为妊娠 C 类药物。这意味着关于该药物的文献有限。目前尚未进行动物生殖研究或对胎儿发育影响的研究。目前尚不清楚该药物是否会损害正在发育的胎儿或损害孕妇的生殖能力。
妊娠30周后,免疫球蛋白可逐渐从母体循环穿过胎盘。妊娠期间应仅在有临床指征时才使用该液体。
Gammagard 哺乳期妇女的液体
尚无研究表明Gammagard会通过母乳排泄。然而,由于许多药物存在进入母乳的风险,因此在服用时应谨慎。 Gammagard 免疫球蛋白 为正在哺乳的人提供解决方案。
了解免疫球蛋白的注意事项将大大提高治疗效果,并有助于预防不良反应。治疗前,请告知医生您的病史和其他相关健康信息。
配方、注射部位和剂量
为了抵抗病毒、细菌或其他异物,人体必须保持足够的抗体水平。Gammagard 液体含有抗体免疫球蛋白 G (IgG),有助于抵抗感染。
IVIG 有帮助吗?
免费 IVIG 治疗信息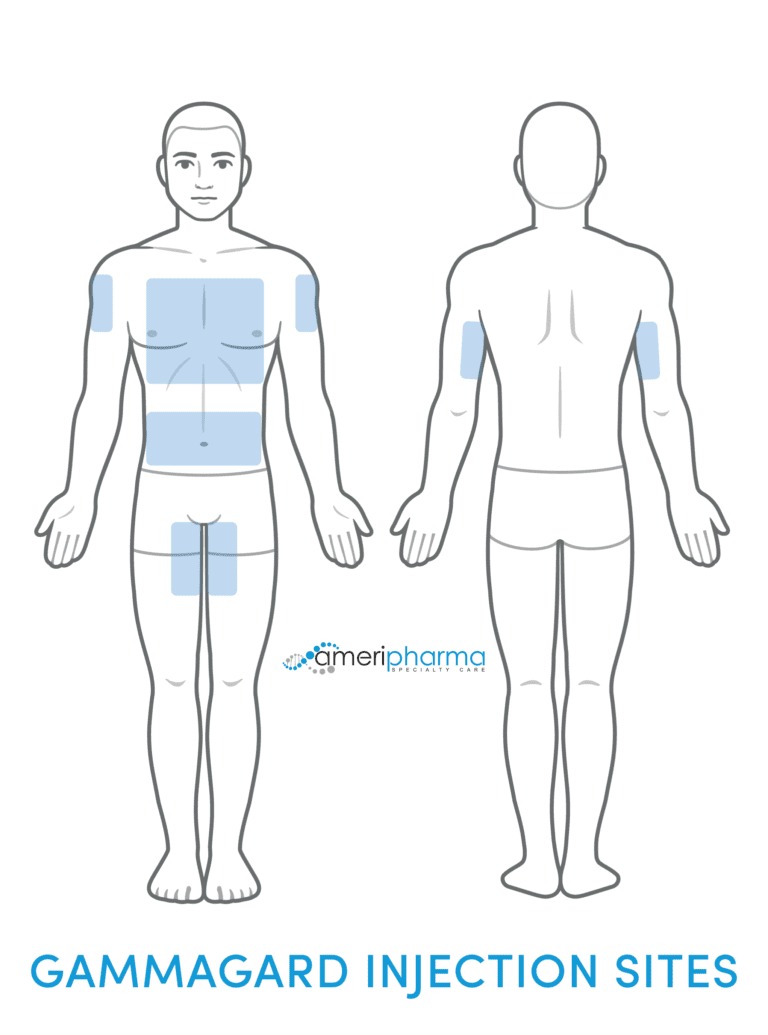
Gammagard 四
- 由训练有素的医疗专业人员将药物直接注射到患者手臂的静脉中(静脉注射)。
- 频率:每3至4周一次。
通常在医院、诊所或患者住所进行。
Gammagard SubQ
- 将针头放入皮下组织(皮肤下的脂肪组织)。
- 通常出现在腹部、大腿或上臂区域。
- 针头可以放置在多个部位(最多 8 个不同部位)。
- 患者通常每周接受一次治疗。
- 经过医疗专业人员的培训后,患者可以独立地自行服用药物。
- 如果患者或护理人员已经熟悉正确的治疗方法并接受过培训,则通常在家中进行这种疗法。
立即获取您的家用 Gammagard IVIG 输液!
剂量
静脉注射PI
每3至4周静脉注射剂量为300至600毫克/公斤。医生会调整剂量 Gammagard 根据患者的临床反应。
初始输注速率:
初始给药速度为0.5 ml/kg/小时(0.8 mg/kg/分钟),持续30分钟。之后可根据患者的耐受性增加给药速度。
维持输注速率:
如果输注耐受性良好,则可以每 30 分钟将输注速度增加至 5 ml/kg/小时(8 mg/kg/分钟)。
MMN 的静脉注射
剂量:
静脉注射剂量范围为每月每公斤体重0.5至2.4克。医生会根据患者的临床反应调整剂量。
初始输注速率:
起始速率为 0.5ml/kg/hr(0.8 mg/kg/min)。
维持输注速率:
如果可以耐受,输注速度可增加至每 30 分钟 5.4 ml/kg/hr(9 mg/kg/min)。
PI 的 SubQ 管理
剂量:
初始剂量为先前静脉注射剂量的1.37倍,除以两次静脉注射之间的周数。维持剂量为 Gammagard 根据患者的临床反应而有所不同。
初始输注速率:
- 如果患者体重超过 40 公斤:初始速率为 30 毫升/部位,之后为 20 毫升/小时/部位。
- 如果患者体重低于 40 公斤:初始速率为 20 毫升/部位,之后为 15 毫升/小时/部位。
维持输注速率:
- 如果患者体重超过 40 公斤:初始注射速度为 30 毫升/部位,之后以 20 至 30 毫升/小时/部位的速率注射。
- 如果患者体重低于 40 公斤:初始速率为 20 毫升/部位,之后为 15 至 20 毫升/小时/部位。
询问 IVIG 家庭输液
Gammagard 用途
当患者的免疫系统受到损害时,他们更容易受到病毒和细菌的侵害。 Gammagard 免疫球蛋白 液体补充个体的抗体水平,使他们拥有能够克服这些敏感性的免疫系统。
在美国,大约 25万人 已被诊断患有原发性免疫缺陷症 (PI)。幸运的是,这种液体有多种关键用途,我们将在下文详细讨论。
维持抵抗感染的免疫力
在一项研究中, Gammagard J 代码 每3至4周给61名原发性免疫缺陷患者注射一次,持续一年。剂量为300 – 600 mg/kg。
研究得出的结论是,这种治疗不会发生细菌感染。 此外,在 61 名患者中,没有人需要因尿路感染、胃肠炎或耳部感染以外的原发性感染而住院。
根据同一项研究,该药物的副作用是可以忍受的。最常见的不良反应是轻微头痛,发生率为5.2%。
原发性免疫缺陷的治疗
PI 是一种免疫反应不足的疾病。它具有遗传性,可影响所有年龄和性别的人。PI 患者需要接受免疫治疗以维持其健康,并且 Gammagard 是治疗这种病症的药物之一。
MMN的管理
免疫球蛋白用于患有 多灶性运动神经病 作为改善肌肉力量和残疾的维持疗法。
Gammagard成本
考虑到它的所有健康益处,这种药物的价格合理。Gammagard 的价格 免疫球蛋白 液体的价格可能因患者就诊的药房或诊所而异。以下是该药物价格的粗略估算:
| 数量 | 单价 | 价格 |
|---|---|---|
| 10毫升 | $19.64 | $196.40 |
| 25毫升 | $19.04 | $476.01 |
| 50毫升 | $18.84 | $942.00 |
| 100毫升 | $18.74 | $1,874.00 |
| 200毫升 | $18.69 | $3,737.99 |
| 300毫升 | $18.67 | $5,601.99 |
其他 IVIG 品牌包括 Gamunex-C 和 PrivigenPrivigen 价格较低,每 100 毫升成本约为 $16。此外,Privigen 的起始剂量仅为 50 毫升。 Gammagard liquid 和 Gamunex 的价格范围相似。
共同支付援助
为治疗原发性皮炎 (PI) 或多发性骨髓瘤 (MMN) 而开具此药的患者,可获得共付额援助 (Copay Assistance)。武田提供一项援助计划,患者可以自行或通过医疗机构加入。 访问我们的 Gammagard 共付额援助 如果您有兴趣获得 Gammagard 的财务援助,请访问此页面。
获得 IVIG 共同支付援助
与专家交谈免疫球蛋白短缺
从 2019 年开始,全球出现免疫球蛋白短缺。这 短缺 由于人体血浆捐献减少,尤其是在 COVID-19 大流行期间。
幸运的是,FDA 已经批准 Gammagard 免疫球蛋白 液体并延长其保质期,从而减少供应短缺的可能性。
提示 Gammagard 治疗
当您在外接受治疗时,请遵循以下提示:
- 带上一本书、一部电影或其他放松的资料,在输液期间打发时间。输液时间通常为2到6小时,具体取决于剂量。
- 放松并舒适地坐着。
- 如果您在输液过程中的任何时候感到不适,请告知护士。
- 告知医生您所经历的任何不良反应。
- 在您的日志中记录您的输液情况。
如果患者正在服用 Gammagard 医学在家进行交流时,至关重要的是:
- 收到产品后,请在使用前目视检查产品是否含有颗粒物和变色。本品为无色或淡黄色溶液,呈澄清或略带乳白色。如果液体浑浊、混浊或含有颗粒物,请勿使用。
- 将小瓶冷藏直至使用。
- 开封后请立即使用,因为药瓶仅供一次性使用。已使用过的部分药瓶请丢弃。
- 让冷藏产品在使用前达到室温。
- 避免用微波炉加热、摇晃或将药物与其他产品混合。
常见问题解答
什么是 Gammagard 用于治疗?
医生使用 Gammagard 免疫球蛋白 该溶液可用于治疗免疫缺陷、自身免疫性疾病、多灶性运动神经病和阿尔茨海默病。此外,该液体还可治疗两岁及以上原发性免疫缺陷患者。
它是一种免疫抑制剂吗?
这种溶液被归类为免疫抑制剂,因为医生开它来治疗自身免疫性疾病。
液体中含有什么抗体?
Gammagard 包含 免疫球蛋白G 免疫球蛋白G(IgG)。这些抗体是从健康个体的血浆中分离和合成的。
何时使用它?
该药物具有多种用途和益处。根据医生开具的治疗方案,可在以下情况下使用: