Xembify is a type of subcutaneous immunoglobulin (SCIG). It contains a broad spectrum of antibodies (immune globulins) extracted from a large pool of human plasma.
Xembify is a 20% immune globulin solution that is FDA indicated for primary humoral immunodeficiency and can be used in a number of other medical conditions.
Get Your Xembify Dose
Speak to a SpecialistWhat Is Inside Xembify?
Xembify is a non-pyrogenic ready-to-use sterile product. The solution contains IgG (18-22% protein) present in 0.16 M to 0.26 M glycine and around 10 – 40 mcg/ml polysorbate.
Xembify does not contain any preservatives and is not made with rubber latex.
What Does It Look Like?
Xembify is colorless to a pale yellow solution that is almost clear or slightly opalescent.
How Does Xembify Work?
Xembify provides a variety of immunoglobulins (Ig) that are opsonizing (more easily destroyed) and neutralizing in nature. Bacteria, viruses, parasites, and mycoplasmal agents and the toxins they release are neutralized by the antibodies found in Xembify.
Xembify Copay Assistance
Xembify Storage
Xembify is stored refrigerated (2 – 8 degrees Celsius). It can also be stored at room temperature for up to 6 months, any time prior to the expiration date. Do not freeze.
If refrigerated, let Xembify come to room temperature before use. Do not microwave.
Treatment/Therapy
Xembify is FDA-indicated for primary humoral immunodeficiency in patients 2 years of age and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency (CVID), X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
Primary Humoral Immunodeficiency (PI)
The suboptimal function of the immune system in a primary humoral immunodeficiency patient can be detrimental. Treatment with Xembify can greatly strengthen the immune system and improve the quality of life.
Contraindications
Although Xembify has a wide window of tolerance and acceptance, there still are some cases where Xembify administration is contraindicated. Some of these conditions are mentioned below.
Allergic Reaction
Xembify must not be given to candidates who have had an anaphylactic or severe allergic reaction to human immune globulins. The major manifestations of this hypersensitive reaction include:
- Difficulty breathing
- Swelling in the neck area
- Severe skin rash
- Acrocyanosis (extremities turning blue)
You should seek medical attention immediately if the symptoms mentioned above start appearing.
If you have experienced such issues previously with IVIG, then you must not use Xembify.
IgA Deficiency
You should stay away from Xembify or any other immunoglobulin infusion if you are IgA deficient. This is because IgA deficient people usually develop anti-IgA antibodies. Therefore, the body responds via a hypersensitivity reaction, which can sometimes be fatal.
Subcutaneous Infusion
Unlike IVIG therapies, which are given intravenously, Xembify is given by subcutaneous route only.
Dosage
A patient’s pharmacokinetics and clinical response determine the dose of Xembify. The following sequence is adopted to determine the right amount.
- After initiating Xembify treatment, measure the serum IgG trough level of the patient as early as 5 weeks.
- Continue monitoring IgG trough level every 2 to 3 months to determine if any subsequent dose adjustments are needed.
- For frequent dosing (i.e., two to seven times per week), divide the calculated weekly dose by the desired number of infusions per week.
Note: If a patient does not maintain adequate clinical response to Xembify therapy (or serum IgG level is not achieved to the level of a previous treatment), dose adjustments may be necessary. For dose adjustments, the doctor will calculate the difference between the IgG trough level from the target IgG trough level, then find the difference via the following table:
Dose Adjustment (Ml per Week)
| Difference from Target IgG trough level (mg/dl) | 10kg | 15kg | 20kg | 30kg | 40kg | 50kg | 60kg | 70kg | 80kg | 90kg | 100kg | 110kg | 120kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 5 |
| 100 | 1 | 1 | 2 | 2 | 3 | 4 | 5 | 5 | 6 | 7 | 8 | 8 | 9 |
| 150 | 1 | 2 | 2 | 3 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 14 |
| 200 | 2 | 2 | 3 | 5 | 6 | 8 | 9 | 11 | 12 | 14 | 15 | 17 | 18 |
| 250 | 2 | 3 | 4 | 6 | 8 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 |
| 300 | 2 | 3 | 5 | 7 | 9 | 11 | 13 | 16 | 18 | 20 | 23 | 25 | 27 |
| 350 | 3 | 4 | 5 | 8 | 11 | 13 | 16 | 19 | 21 | 24 | 27 | 29 | 32 |
| 400 | 3 | 5 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 |
| 450 | 3 | 5 | 7 | 10 | 14 | 17 | 20 | 24 | 27 | 31 | 34 | 38 | 41 |
| 500 | 4 | 6 | 8 | 11 | 15 | 19 | 23 | 27 | 30 | 34 | 38 | 42 | 45 |
Locate the corresponding amount (in ml) by which to increase/decrease the weekly dose based on the patient’s body weight.
Example: If a patient with a body weight of 70 kg has an IgG trough level of 900 mg/dl and the target level is 1000mg/dl, the difference is 100mg/dl. Thus, increase the weekly dose by 5 ml.
Safe Xembify Administration
Get Prior Authorization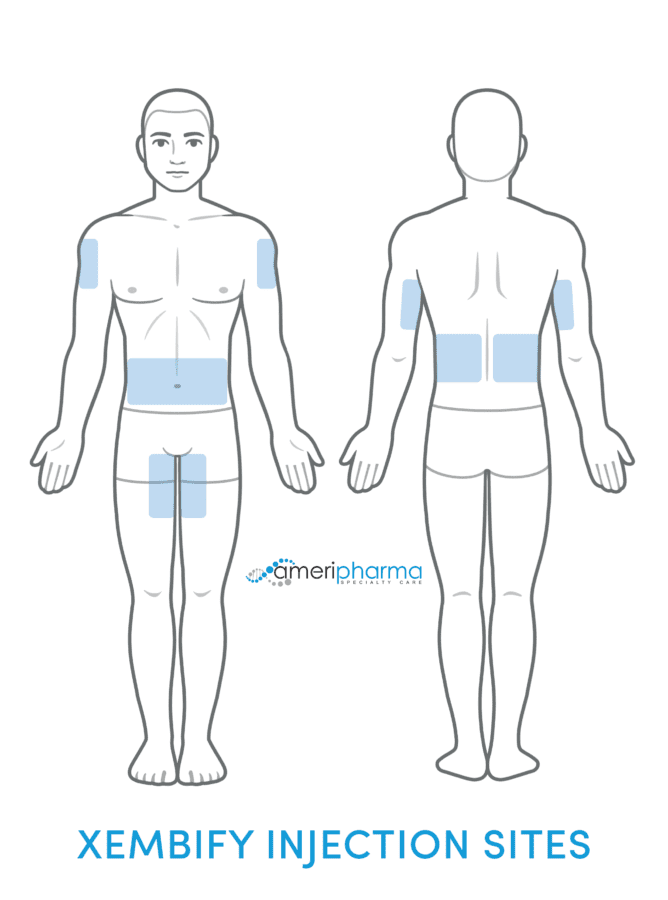
Precautions
Pregnancy
No human data is available to indicate the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with Xembify. It is not known whether it can cause fetal harm when administered to a pregnant woman or if it can affect reproduction capacity.
Lactating Mothers
No human data is available to indicate the presence or absence of drug-associated risk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Xembify and any potential adverse effects on the breastfed infant.
Pediatric Use
Xembify was evaluated in 14 pediatric subjects (2 – 16 years of age) with PI in a multi-center clinical trial. The safety and efficacy profiles were similar to adult subjects. No pediatric-specific dose requirements were necessary to achieve the desired serum IgG levels. The safety and effectiveness of Xembify in pediatric patients below 2 years of age have not been established.
Geriatric Use
Clinical studies of Xembify did not include sufficient numbers of subjects over age 65 to determine whether they respond differently from younger subjects. Three study subjects enrolled in the clinical trial were 65 years and older. In general, dose selection for an elderly patient should be made cautiously, usually starting at the low end of the dosing range.
Cost
The cost of Xembify is around $188 for a supply of 5 ml.
Get Your IVIG Dose
At-Home InfusionCopay Assistance
Copay assistance programs are available for Xembify, where eligible patients may pay $0. The programs offer copay assistance ranging from $5,000 to $10,000 per year.
A patient will need to apply to see if they meet the criteria to receive copay assistance.
Warnings of Xembify Usage
Upon evaluation, your doctor will determine if Xembify is ideal for you. Inform your doctor if you:
- Have cardiovascular (heart) problems
- Suffer from hyperviscosity (thickness) of blood from a sedentary lifestyle
- Have a permanent IV catheter placed
- Are taking any medications that contain estrogen
- Have renal (kidney) impairment
- Have risk factors of thrombosis
Patients with the risk of thrombosis should be treated with extra caution. In such cases, the doctor may prescribe Xembify at a minimum dose and a lower infusion rate.
Warnings and Precautions
Hypersensitivity Reactions
Severe hypersensitivity reactions may occur with human immune globulin products, including Xembify. If a hypersensitivity reaction occurs, discontinue the Xembify infusion immediately and deliver appropriate treatment.
Xembify contains IgA. Patients with known anti-IgA antibodies have a greater risk of developing potentially severe hypersensitivity and/or anaphylactic reactions. Xembify is contraindicated in IgA deficient patients with antibodies against IgA and a history of hypersensitivity to human immune globulin treatment.
Thrombosis
Thrombosis may occur following treatment with immune globulin products, including Xembify.
Risk Factors:
- Advanced age
- Prolonged immobilization
- Hypercoagulable conditions
- History of venous/arterial thrombosis
- Use of estrogens
- Indwelling central vascular catheters
- Hyperviscosity
- Cardiovascular factors
Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triglycerides, or monoclonal gammopathies. For patients at risk of thrombosis, administer Xembify at the minimum dose and infusion rate practicable.
Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
Aseptic Meningitis Syndrome (AMS)
AMS has been reported with the use of human immune globulin administered intravenously and subcutaneously. It usually begins within several hours to 2 days following immune globulin treatment. AMS may occur more frequently in females than in males.
Symptoms:
- Severe headache
- Nuchal rigidity (neck stiffness)
- Drowsiness
- Fever
- Photophobia (sensitivity to light)
- Painful eye movements
- Nausea
- Vomiting
Cerebrospinal fluid (CSF) studies frequently show pleocytosis up to several thousand cells per cubic millimeter, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dl, but negative culture results.
To rule out other causes of meningitis, conduct a thorough neurological examination on patients exhibiting symptoms, including CSF studies.
AMS may occur more frequently in association with high doses (>2 g/kg) and/or rapid infusion of immune globulin products. Discontinuation of immune globulin treatment has resulted in remission of AMS within several days without sequelae (after-effects).
Get IVIG Copay Assistance
IVIG Financial AssistanceRenal Failure and Hemolysis
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur upon the use of human immune globulin products, especially those containing sucrose. Xembify does not contain sucrose. Ensure that patients are not volume depleted prior to administration of Xembify.
Patients at Risk
Patients at risk of developing renal dysfunction include patients with any degree of preexisting renal insufficiency or predisposition to acute renal failure, such as:
- Diabetes mellitus
- Age over 65
- Volume depletion
- Sepsis
- Paraproteinemia
- Receiving known nephrotoxic drugs
For such patients, monitor renal function and consider lower, more frequent dosing.
Periodic monitoring of renal function and urine output is particularly important in patients who have a potential increased risk for developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN)/serum creatinine, prior to the initial infusion and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of Xembify.
Hemolysis
IgG products, including Xembify, can contain blood group antibodies that may act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin (Coombs’) test result and hemolysis. Delayed hemolytic anemia can develop subsequent to human immune globulin therapy due to enhanced RBC sequestration, and acute hemolysis consistent with intravascular hemolysis has been reported.
Monitor Xembify recipients for clinical signs and symptoms of hemolysis, particularly patients with risk factors such as non-O blood group or patients receiving high IgG doses (≥ 2 g/kg). Underlying inflammatory states in an individual patient may increase the risk of hemolysis, but its role is uncertain.
If signs and/or symptoms of hemolysis are present after Xembify infusion, perform appropriate confirmatory laboratory testing.
Transfusion-Related Acute Lung Injury (TRALI)
Noncardiogenic pulmonary edema may occur in patients following treatment with human immune globulin products. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with ventilatory support.
Interference With Lab Tests
After infusion with Xembify, the transitory rise of various passively transferred antibodies in the patient’s blood may yield false-positive serological testing results, with the potential for misleading interpretation. Be sure to inform your doctor about your therapy.
Transmission of Infectious Agents
Because Xembify is made from human blood, it may carry the risk of transmitting infectious agents (i.e., viruses), the variant Creutzfeldt-Jakob disease (vCJD) agent, and the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
No cases of transmission of CJD or any other viral disease have been reported with the use of Xembify.
Non-Serious Side Effects
Local reactions to Xembify administration include:
- Infusion site erythema (redness)
- Infusion site pain
- Infusion site swelling
- Infusion site bruising
- Infusion site pruritus (itching)
- Infusion site induration (firmness)
- Infusion site scab
- Infusion site edema
- Systemic reactions (e.g., cough and diarrhea)
The most common adverse reactions in over 5% of subjects in the clinical trial were local adverse reactions.
Ask an IVIG Specialist About Side Effects
Drug Interactions
Live Attenuated Virus Vaccines
Passive transfer of antibodies can transiently interfere with the immune response of the body to live virus vaccines such as:
- Mumps
- Measles
- Rubella
- Varicella
You should inform your healthcare provider of any recent Xembify therapy before getting vaccinated so that appropriate precautionary steps can be taken.
Serological Testing
Various passively transferred antibodies in immunoglobulin preparations, including Xembify, can confound the results of serological testing.