
Ilumya 注射剂已获得 FDA 批准,用于治疗患有中度至重度 斑块状银屑病。如果您或您所爱的任何人患有斑块状银屑病,本文将介绍您需要了解的有关这种药物的所有信息,包括其用途、作用机制、剂量、副作用和费用。
获得经济援助
重要 Ilumya 警告
感染风险增加
此药物可能会抑制您的免疫系统,增加感染风险。开始治疗前,您的医疗保健提供者会安排检查,以确定您是否患有感染或结核病 (TB)。
如果您患有活动性结核病或过去曾患过结核病,您的医疗保健提供者可能会在开始治疗之前对您进行结核病治疗。
如果您出现以下感染迹象,请立即致电您的医疗保健提供者:
- 发烧、出汗或发冷
- 咳嗽
- 气促
- 粘液中带血
- 肌肉酸痛
- 皮肤发热、发红或疼痛,或出现非牛皮癣引起的溃疡
- 体重减轻
- 腹泻
- 胃痛
- 排尿时有灼烧感
- 尿量增加
参加 Ilumya 之前
在您接种第一剂之前,请告知您的医疗保健提供者您是否有以下情况:
- 对本产品或任何产品成分有过敏史。
- 患有长期(慢性)或复发性感染。
- 患有结核病,或者您身边的人被诊断出患有结核病。
- 近期已接种疫苗或计划接种疫苗。治疗期间不应接种活疫苗。活疫苗包括MMR(麻疹、腮腺炎、风疹)、水痘疫苗和鼻喷流感疫苗。
Ilumya简介及用途
Ilumya 是品牌处方药。暂无仿制药。
该产品的活性成分是 tildrakizumab-asmn,属于单克隆抗体类靶向药物疗法。
单克隆抗体是实验室产生的免疫系统蛋白质,可以抑制、模仿或增强您的免疫反应。
2018年3月,美国FDA批准该产品用于治疗中度至重度斑块状银屑病。对于可能受益于全身治疗(口服或注射药物)或光疗法的成年人,可考虑接受Ilumya疗法。
斑块性银屑病是一种 自身免疫性疾病 其特征是肘部、背部、膝盖和头皮上出现厚厚的鳞状斑块。在严重的情况下,斑块可能会影响全身。
立即获得共付额援助
Ilumya作用机制
Ilumya 直接阻断 IL-23 蛋白的作用,而 IL-23 在炎症和免疫反应中起着至关重要的作用。通过阻断 IL-23,Ilumya 能够:
- 帮助平衡过度活跃的免疫反应。
- 减少皮肤中炎症细胞的积聚。
- 阻止鳞状斑块的形成。
皮肤因此变得更清爽,炎症减少,瘙痒感也减轻。临床研究表明,近60%使用Ilumya的患者在3个月后症状消失或极轻微。
Ilumya剂量
这种药物是透明至微黄色的液体,可注射到皮下(皮下/SC)。
美国提供以下强度的药物:单剂量预充式注射器中的 100 mg/ml 溶液。
您的医疗保健提供者或护士会将Ilumya注射剂注射到您的胃部、大腿或上臂。首剂为100毫克皮下注射。4周后,第二剂为100毫克皮下注射。之后,每12周皮下注射100毫克。
笔记: 您不应自行注射 Ilumya。
Ilumya 副作用
常见副作用
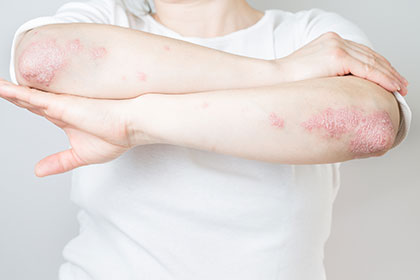
如果以下症状恶化或没有消失,请致电您的医疗保健提供者。
- 上呼吸道感染
- 注射部位反应(注射部位疼痛、肿胀或出血)
- 腹泻
过敏反应
如果您遇到以下情况,请寻求紧急医疗护理:
- 感觉自己快要晕过去了
- 面部、眼睑、嘴唇、口腔、舌头或喉咙肿胀
- 皮疹
- 呼吸困难
- 喉咙或胸口紧绷
怀孕和哺乳期间使用
由于关于孕妇使用 Ilumya 的数据有限,因此无法确定该药物是否会损害胎儿。此外,也不清楚该药物是否会增加女性受孕难度。
Ilumya 病毒可能从母亲传染给胎儿。然而,其对母亲或胎儿的影响尚不清楚。
动物生殖研究并未报告怀孕期间使用会增加流产或出生缺陷的风险。[1].
目前尚无关于哺乳期使用的数据。由于替达珠单抗是一种大分子蛋白质,因此不太可能进入母乳。然而,动物研究中检测到了该药物在母乳中的存在。
漏服剂量
如果您错过了与医疗保健提供者预约的 Illumya 剂量,请尽快安排另一个预约。
过量
如果服用过量,请寻求紧急医疗救助或拨打中毒帮助热线 1-800-222-1222。
与专家交谈
关于共付额援助Ilumya成本
1 个单位(100 mg/ml SC 溶液)的价格约为 $19,000。
但是,费用可能会因您的保险计划、所在地和药房而异。请联系您的保险公司,了解您的保险计划是否涵盖此药物,或者是否需要事先授权。
如果您符合条件,Ilumya 的制造商 Sun Pharmaceutical Industries, Inc. 将为您提供财务支持。
拥有商业保险的人可以参加 ILUMYA SUPPORT LIGHTING THE WAY® 计划并使用共同支付卡支付每剂 $0。
对于没有商业保险的人,ILUMYA 支持照亮前路® 可以通过将他们与专门的支持专家联系起来提供帮助。
对于拥有 Medicare B 部分补充保险的人来说,处方费用可能为 $0。
联系我们 如果您有兴趣了解 Ilumya 的财务援助选项。
Ilumya 与 Skyrizi
虽然 Ilumya 和 Skyrizi 都用于治疗中重度斑块状银屑病,但它们在几个方面存在差异。以下是一些区别:
| Ilumya | Skyrizi | |
| FDA批准日期 | 2018 | 2019 |
| 活性药物 | 替达珠单抗 | 瑞桑基珠单抗 |
| 谁可以管理? | 仅限医疗保健专业人员 | 训练后自我管理 |
| 可用强度 | 100毫克 | 75毫克 |
| 批准用途 | 成人中度至重度斑块性银屑病 | 中度至重度斑块性银屑病、活动性银屑病关节炎、 克罗恩病以及成人溃疡性结肠炎 |
参考:
- MotherToBaby | 情况说明书 [互联网]。布伦特伍德(田纳西州):畸形学信息专家组织 (OTIS);1994-。Tildrakizumab (Ilumya®) 2023年2月。获取网址:https://www.ncbi.nlm.nih.gov/books/NBK582978/













