血清阴性重症肌无力 (SnMG) 是指患者血液中缺乏常见的可检测抗体,例如乙酰胆碱受体 (AChR) 和肌肉特异性受体酪氨酸激酶 (MuSK),而这些抗体正是导致重症肌无力的原因。血清阴性重症肌无力是一种罕见疾病,通常无法确诊。
与专家讨论共付额援助
一般来说,当患者被诊断患有重症肌无力(MG)时,血液中会存在可检测到的乙酰胆碱受体(AChR)抗体或抗麝香蛋白(MuSK)抗体。然而,对于特发性重症肌无力(SnMG),患者血液中没有乙酰胆碱受体(AChR)或抗麝香蛋白(MuSK)抗体,但仍会出现重症肌无力症状,例如眼睑下垂、视力模糊以及呼吸、吞咽和说话困难。
事实上,大致 10-20% 的重症肌无力患者被诊断为血清阴性。然而,实际数字可能更高,因为许多血清阴性重症肌无力患者很可能没有得到准确的诊断。
本文讨论了血清阴性 MG 的基本方面,包括血清阴性和血清阳性 MG 之间的区别、亚型、症状、诊断以及可以考虑的血清阴性 MG 的治疗方法。
血清阴性和血清阳性重症肌无力之间的区别
医疗保健提供者考虑区分血清阴性和血清阳性重症肌无力的共同因素是检查患者血液中是否存在乙酰胆碱受体 (AChR) 或肌肉特异性受体酪氨酸激酶 (MuSK) 抗体。
大约 80-90% 确诊患有重症肌无力 (MG) 的患者血液中含有乙酰胆碱受体 (AChR) 抗体。乙酰胆碱受体 (AChR) 是重症肌无力患者中检测到的第一种抗体。
以前,当 MG 患者未检测到 AChR 抗体时,他们就被认为患有 血清阴性MG然而,随着更多研究的深入,人们发现低密度脂蛋白受体相关蛋白4(LRP4)参与了神经肌肉接头处的工作。
简而言之,患者 血液中没有 AChR 或抗 MuSK 自身抗体 被诊断为血清阴性 MG,而具有 AChR 或抗 MuSK 自身抗体的患者被诊断为血清阳性 MG。
血清阴性重症肌无力亚型
各种文献对血清阴性 MG 疾病进行了进一步分类 根据抗体的存在与否分为两种亚型:
双血清阴性MG
在双重血清阴性 MG 中,患者血液中均不存在 AChR 和 MuSK 抗体。
三重血清阴性重症肌无力
在三重血清阴性 MG 中,患者血液中不存在 AChR、MuSK 和 LRP4 抗体。
血清阴性重症肌无力(SnMG)的症状是什么?
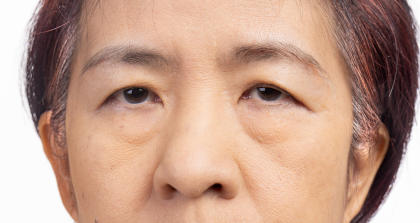
SnMG 的症状与血清阳性 MG 相同。与血清阳性 MG 一样,血清阴性 MG 可表现为眼部或全身症状。然而, 血清阴性重症肌无力患者眼部重症肌无力的发病率较高 重症肌无力患者比血清阳性患者更易出现症状,症状可轻可重。常见症状包括:
- 眼睑下垂
- 视力模糊或复视
- 咀嚼、吞咽和说话困难
- 面部肌肉无力
- 呼吸困难(严重情况下)
重复活动会使症状恶化,休息后症状会改善。
IVIG 有帮助吗?| 免费 IVIG 治疗信息 | 诊断困难?
如何诊断血清阴性重症肌无力(SnMG)?
与血清阳性 MG 相比,诊断血清阴性 MG 患者需要一套更先进的诊断技能。
通常,重症肌无力专科医生会彻底检查患者的病史和症状严重程度,并进行筛查测试,包括:
- 血液检测是否存在 AChr、MuSK 和 LRP4 自身抗体
- 肌电图(EMG)
- 重复神经刺激(RNS)
- 单纤维肌电图(SFEMG)
最近,一种名为细胞分析(CBA)的新技术被发现有助于诊断血清阴性患者。 学习 发表在《神经免疫学杂志》上的一项研究报告称,当99名血清阴性重症肌无力患者接受基于细胞的检测(CBA)时,18名患者的乙酰胆碱受体(AChR)检测呈阳性。这些发现支持使用聚集性乙酰胆碱受体CBA检测来诊断血清阴性重症肌无力。
同样地,另一个 学习 报告了相同的结果,并强调了对血清阴性 MG 患者进行 CBA 的诊断价值。
大多数情况下,重症肌无力检测呈阴性的患者往往会被医疗专业人员忽视,这使他们面临接受速度过慢的劣质护理的风险。
血清阴性重症肌无力(SnMG)的治疗方案有哪些?
血清阴性重症肌无力 (MG) 患者在接受治疗时往往面临挑战。即使接受治疗,他们也可能无法获得足够的护理来改善症状。由于 SnMG 难以诊断,治疗时间可能比其他疾病更长。
目前,市场上大多数经FDA批准的新疗法仅适用于血清阳性的重症肌无力患者。然而,血清阴性的重症肌无力患者可以在医疗保健提供者的监督下尝试以下治疗方案:
- 药物(例如,美斯汀)
- 皮质类固醇(例如泼尼松等)
- 静脉注射免疫球蛋白(IVIG) 或者 皮下注射免疫球蛋白(SCIG)
- 血浆置换术(PLEX)
- 免疫抑制治疗
- 胸腺切除术
目前,针对血清阴性重症肌无力 (SnMG) 的研究正在进行中。研究人员仍在努力了解哪些类型的抗体或蛋白质与此类重症肌无力有关,以便为患者制定精准的诊断和治疗方案。
参考:
- Vinciguerra, C.、Bevilacqua, L.、Lupica, A.、Ginanneschi, F.、Piscosquito, G.、Rini, N.、Rossi, A.、Barone, P.、Brighina, F. 和 Stefano, VD (2023)。血清阴性重症肌无力的诊断和治疗:光与影。 脑科学, 13(9)。https://doi.org/10.3390/brainsci13091286
- Romi, F.、Aarli, JA 和 Gilhus, NE (2005)。血清阴性重症肌无力:疾病严重程度和预后。 欧洲神经病学杂志, 12(6), 413–418。https://doi.org/10.1111/j.1468-1331.2005.01137.x
- Birmanns, B., Brenner, T., Abramsky, O., & Steiner, I. (1991).血清阴性重症肌无力:临床特征、治疗反应及体外乙酰胆碱受体抗体的合成。 神经科学杂志, 102(2), 184-189. https://doi.org/10.1016/0022-510X(91)90067-H
- Romi, F.、Aarli, JA 和 Gilhus, NE (2005)。血清阴性重症肌无力:疾病严重程度和预后。 欧洲神经病学杂志, 12(6), 413-418. https://doi.org/10.1111/j.1468-1331.2005.01137.x
- Lazaridis, K., & Tzartos, SJ (2020).重症肌无力:自身抗体特异性及其在重症肌无力管理中的作用。 神经病学前沿, 11. https://doi.org/10.3389/fneur.2020.596981
- Vincent, A., McConville, J., Farrugia, M., & Newsom-Davis, J. (2004).血清阴性重症肌无力。 神经病学研讨会, 24(01), 125–133。https://doi.org/10.1055/s-2004-829589
- Evoli, A.、Tonali, PA、Padua, L.、Monaco, ML、Scuderi, F.、Batocchi, AP、Marino, M. 和 Bartoccioni, E. (2003)。与全身性血清阴性重症肌无力中的抗 MuSK 抗体的临床相关性。 脑, 126(10), 2304-2311. https://doi.org/10.1093/brain/awg223
- 血清阴性重症肌无力资源中心 | MGFA。https://myasthenia.org/Understanding-MG/Seronegative-MG-Resource-Center
- Methods for diagnosing seronegative myasthenia gravis. (2008). PubMed. https://pubmed.ncbi.nlm.nih.gov/18368681/#:~:text=Development%3A%20Its%20diagnosis%20requires%20the,testing%20of%20the%20neuromuscular%20junction
- Cortés-Vicente, E.、Gallardo, E.、Martínez, M. Á.、Díaz-Manera, J.、Querol, L.、Rojas-García, R. 和 Illa, I. (2016)。双血清阴性重症肌无力和皮质素抗体患者的临床特征。 《JAMA神经病学》, 73(9), 1099. https://doi.org/10.1001/jamaneurol.2016.2032
- Masi, G., Li, Y., Karatz, T., Pham, MC, Oxendine, SR, Nowak, RJ, Guptill, JT, & O'Connor, KC (2022). 血清阴性重症肌无力(MG)患者对聚集性乙酰胆碱受体(AChR)细胞检测的临床需求。 神经免疫学杂志, 367, 577850. https://doi.org/10.1016/j.jneuroim.2022.577850