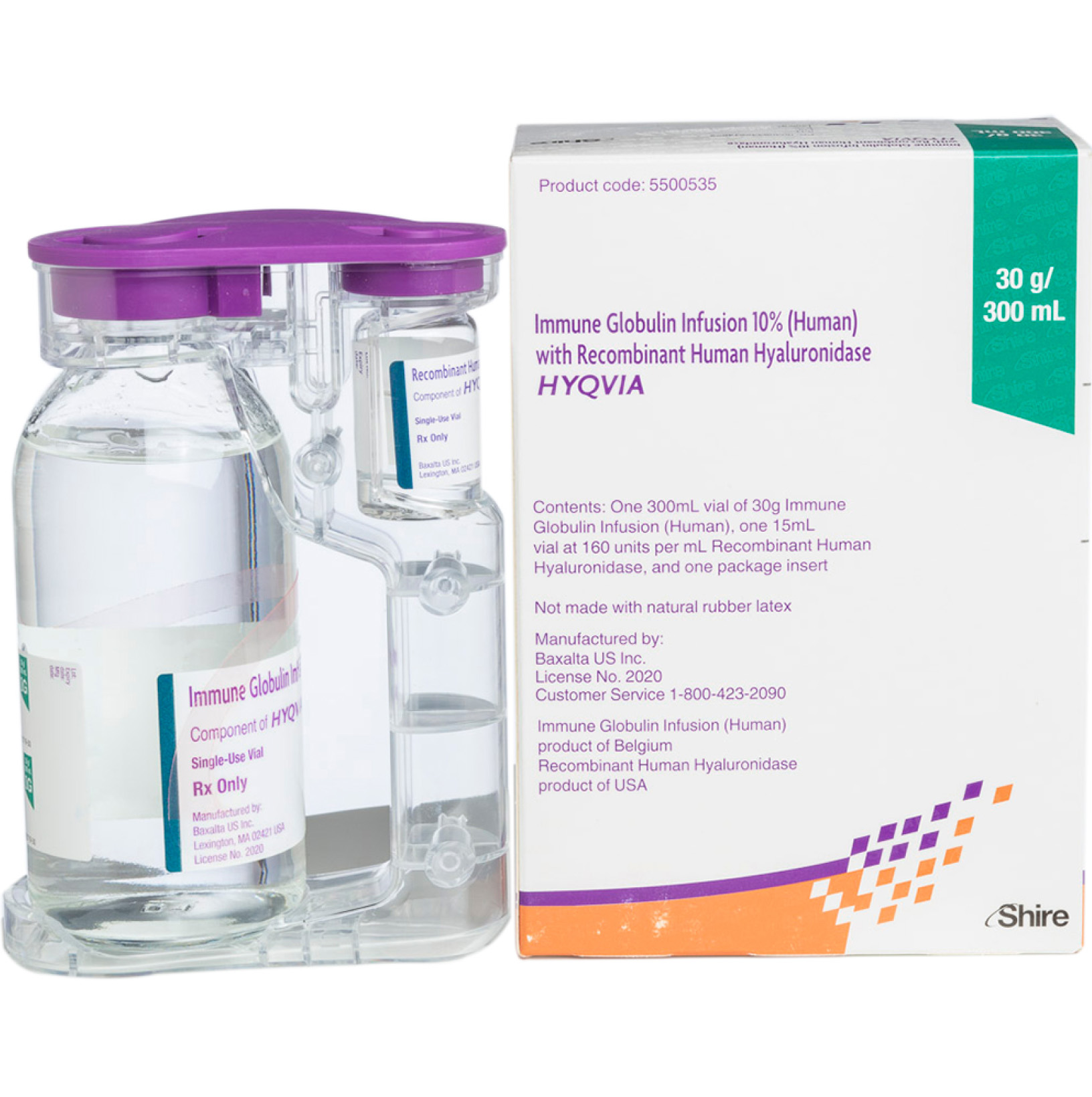
Tên thương hiệu: Hyqvia
Nhà sản xuất: Tập đoàn chăm sóc sức khỏe Baxter, Baxter BioScience
Các lựa chọn thay thế: Truyền globulin miễn dịch 10% với hyaluronidase người tái tổ hợp
Nhận liều Hyqvia của bạn
Nói chuyện với một chuyên giaHyqvia là gì?
Hyqvia là một loại thuốc tiêm chứa một protein được thiết kế di truyền. Nó là một đơn vị lọ kép với một lọ chứa globulin miễn dịch dịch truyền 10% (Ig) và một lọ khác chứa hyaluronidase người tái tổ hợp (Hy), giúp cơ thể bạn hấp thụ thuốc tiêm.
Các bộ phận được truyền lần lượt (Hy trước, Ig sau) qua một bộ kim.
Điều trị/Liệu pháp
Hyqvia là một loại dịch truyền globulin miễn dịch. Nó là một dung dịch vô trùng được tạo thành từ huyết tương người có chứa kháng thể giúp cơ thể bạn tự bảo vệ chống lại các bệnh truyền nhiễm khác nhau. Nó được sử dụng cho:
- Điều trị suy giảm miễn dịch nguyên phát ở người lớn và bệnh nhi từ hai tuổi trở lên
- Liệu pháp duy trì cho người lớn với bệnh đa dây thần kinh viêm mãn tính mất myelin (CIDP).
Truyền dịch
Hyqvia được dùng dưới dạng tiêm truyền dưới da. Nó có quản lý linh hoạt các lựa chọn và nhà cung cấp dịch vụ chăm sóc sức khỏe có thể hướng dẫn bạn cách tự thực hiện tại nhà một cách thoải mái.
Điều quan trọng là bạn phải được đào tạo bài bản từ chuyên gia chăm sóc sức khỏe và hiểu rõ hướng dẫn trước khi tự dùng thuốc. Không tự ý dùng thuốc nếu bạn không hiểu rõ tất cả các hướng dẫn.
Dịch truyền này cũng có thể được truyền tại trung tâm truyền dịch.
Kho
- Hyqvia có thể được lưu trữ trong tủ lạnh ở 36°F đến 46°F trong tối đa 3 năm (không đông lạnh thuốc).
- Nó có thể được lưu trữ tại nhiệt độ phòng (lên đến 77°F) trong tối đa 3 tháng, nhưng chỉ trong vòng 24 tháng đầu tiên kể từ ngày sản xuất được in trên hộp.
- Không trả lại Hyqvia vào tủ lạnh nếu bạn lấy nó ra và bảo quản ở nhiệt độ phòng.
- Bảo quản Hyqvia trong hộp ban đầu để tránh ánh sáng.
- Sản phẩm được làm lạnh phải được được phép đạt đến nhiệt độ phòng trước chúng tae.
- Không hâm nóng hoặc quay sản phẩm bằng lò vi sóng.
Sự quản lý
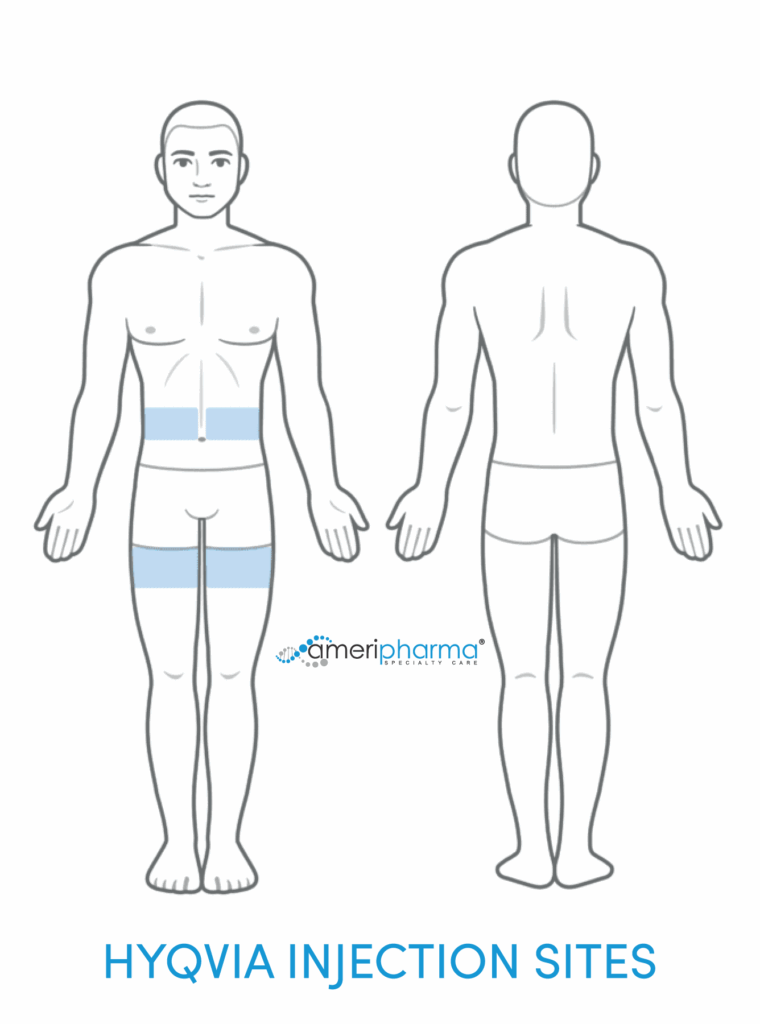
Để truyền dịch, cần có bơm truyền dịch để điều chỉnh lưu lượng thích hợp. Để tránh rò rỉ tại vị trí truyền dịch trong khi sử dụng Hyqvia, cần kim dài hơn (12 hoặc 14 mm) và việc sử dụng nên cân nhắc nhiều vị trí truyền dịch.
- Trước khi truyền, kiểm tra các lọ cho bất kỳ kết tủa đục nào, và không lắc lọ thuốc.
- Không sử dụng thuốc nếu giải pháp không rõ ràng.
- Mỗi lọ chỉ sử dụng một lần và không nên sử dụng quá một lần.
- Các vị trí truyền dịch được đề xuất là đùi hoặc bụng.
- Khi truyền, không trộn lẫn hai loại thuốc được cung cấp trong bộ dụng cụ Hyqvia.
Truyền dịch tuần tự
Cả hai thành phần của Hyqvia đều được truyền tuần tự, bắt đầu đầu tiên với Hyaluronidase, sau đó Ig 10% trong vòng 10 phút thông qua cùng một kim tiêm dưới da.
Các toàn bộ nội dung của lọ Hyaluronidase được sử dụng cho mỗi lọ Ig đầy đủ hoặc một phần.
Phản ứng tại chỗ tiêm truyền như đau nhẹ đến trung bình, sưng mềm tạm thời như bánh kếp, ngứa và đỏ da là những phản ứng thường gặp.
Sưng có thể kéo dài từ 1 đến 3 ngày, và các phản ứng liên quan đến truyền dịch sẽ biến mất sau vài giờ.
Hyqvia Có hỗ trợ đồng thanh toán
Chống chỉ định
Không sử dụng Hyqvia Nếu bạn đã từng bị phản ứng dị ứng với globulin miễn dịch, albumin người và hyaluronidase hoặc nếu bạn thiếu hụt immunoglobulin A.
Cảnh báo và Phòng ngừa
Hyqvia có thể gây ra cục máu đông, đặc biệt nếu bạn từ 65 tuổi trở lên, mắc bệnh tim, bệnh tuần hoàn hoặc có tiền sử bị cục máu đông.
Hãy liên hệ ngay với bác sĩ nếu bạn đột nhiên bị đau ngực, tê liệt, nói không rõ, rối loạn thị lực, khó thở hoặc đau, nóng hoặc sưng ở chân hoặc tay.
Hãy thông báo cho chuyên gia chăm sóc sức khỏe của bạn trước khi truyền dịch nếu bạn có bất kỳ tình trạng sức khỏe nào khác như tiểu đường, bệnh thận, mang thai hoặc mất nước. Không truyền vào hoặc xung quanh vùng bị nhiễm trùng vì có thể khiến nhiễm trùng lây lan.
Tương tác thuốc
Hyqvia có thể tương tác và can thiệp vào phản ứng miễn dịch với vắc-xin virus sống, chẳng hạn như vắc-xin phòng sởi, quai bị, rubella (MMR) và thủy đậu.
Do đó, bạn nên thông báo cho nhà cung cấp dịch vụ chăm sóc sức khỏe về phương pháp điều trị Hyqvia của mình trước khi tiêm bất kỳ loại vắc-xin nào.
Tác dụng phụ
Theo các thử nghiệm lâm sàng, hơn 5% đối tượng đã trải qua một số triệu chứng chung tác dụng phụbao gồm đau đầu, sốt, nôn mửa, buồn nôn, mệt mỏi, hình thành kháng thể kháng hyaluronidase và các triệu chứng dị ứng như sưng, ngứa, đau và phát ban da. Cho đến nay vẫn chưa có bằng chứng về tổn thương da do Hyqvia.
Hyqvia có thể gây ra cục máu đông hoặc huyết khối. Hãy đến cơ sở y tế ngay lập tức nếu bạn gặp bất kỳ tác dụng phụ nào sau đây:
- Các vấn đề về lời nói và thị giác
- Đột nhiên tê liệt và uể oải
- Đau ngực
- Có máu trong ho
- Nước tiểu sẫm màu
- Giảm tiểu tiện
- Vàng da
- Sự nhiễm trùng
- Nhịp tim nhanh
- Đông máu ở cánh tay hoặc chân
- Bệnh phổi như tổn thương phổi cấp tính liên quan đến truyền máu (TRALI)
Có sẵn dịch truyền tại nhà
Liều lượng
Các liều lượng của Hyqvia được thực hiện trên cơ sở cá nhân hóa.
Liều lượng Hyqvia các biến thể được thảo luận dưới đây:
- Nếu bệnh nhân đã truyền Ig, việc điều trị nên được bắt đầu 1 tuần sau lần truyền Ig cuối cùng.
- Tăng dần liều lượng và tần suất từ liều dùng 1 tuần lên liều dùng 3 hoặc 4 tuần.
- Liều khởi đầu là 7,5 g vào tuần 1, 15 g vào tuần 2, 22,5 g vào tuần 4, sau đó là 30 g vào tuần 7 (thông tin đầy đủ có trong tờ hướng dẫn sử dụng Hyqvia).
- Nếu bệnh nhân đã chuyển từ Điều trị IVIG, sau đó dùng Hyqvia với liều lượng và tần suất tương tự như phương pháp điều trị IV trước đó sau khi tăng liều ban đầu.
- Nếu bệnh nhân đã chuyển từ Điều trị SCIG hoặc chưa từng tiêm IgG, sau đó dùng liều 300 – 600 mg/kg cách nhau 3 đến 4 tuần sau khi tăng liều ban đầu.
- Nếu bệnh nhân có nguy cơ cao bị suy thận hoặc huyết khối, hãy dùng Hyqvia ở liều lượng và tốc độ truyền tối thiểu có thể thực hiện được.
Khối lượng trên mỗi trang web:
Có thể tiêm tối đa 600 ml cho mỗi vị trí tiêm đối với bệnh nhân có cân nặng từ 40 kg trở lên và tối đa 300 ml cho mỗi vị trí tiêm đối với bệnh nhân có cân nặng dưới 40 kg.
Tốc độ truyền dịch:
Tiêm hyaluronidase Hyqvia (HY) với tốc độ ban đầu cho mỗi vị trí là khoảng 1 đến 2 ml mỗi phút hoặc tùy theo khả năng dung nạp.
Truyền thành phần globulin miễn dịch 10% (IG) của Hyqvia theo liều lượng được hiển thị bên dưới cho lần truyền ban đầu. Dược sĩ có thể điều chỉnh liều lượng này tùy theo khả năng dung nạp.
| 2 lần truyền đầu tiên | 2 hoặc 3 lần truyền tiếp theo | |||
|---|---|---|---|---|
| Đối tượng < 40 kg (< 88lbs) | Đối tượng ≥ 40 kg (≥ 88lbs) | Đối tượng < 40 kg (< 88lbs) | Đối tượng ≥ 40 kg (≥ 88lbs) | |
| Khoảng thời gian (phút) | Tỷ lệ cho mỗi trang web (ml mỗi giờ) | Tỷ lệ cho mỗi trang web (ml mỗi giờ) | Tỷ lệ cho mỗi trang web (ml mỗi giờ) | Tỷ lệ cho mỗi trang web (ml mỗi giờ) |
| 5 – 15 | 5 | 10 | 10 | 10 |
| 5 – 15 | 10 | 30 | 20 | 30 |
| 5 – 15 | 20 | 60 | 40 | 120 |
| 5 – 15 | 40 | 120 | 80 | 240 |
| Phần còn lại của dịch truyền | 80 | 240 | 160 | 300 |
Công dụng
Hyqvia chủ yếu được chỉ định để điều trị suy giảm miễn dịch nguyên phát (PI) ở người lớn và trẻ em từ 2 tuổi trở lên. Thuốc cũng được sử dụng như liệu pháp duy trì cho bệnh đa dây thần kinh mất myelin do viêm mạn tính (CIDP) ở người lớn.
Các tình trạng suy giảm miễn dịch có thể bao gồm suy giảm miễn dịch biến đổi thông thường, hội chứng Wiskott-Aldrich, bẩm sinh hoặc Bệnh thiếu gammaglobulin liên kết Xvà tình trạng suy giảm miễn dịch kết hợp nghiêm trọng.
Chi phí Hyqvia
Chi phí trung bình cho dung dịch tiêm dưới da Hyqvia (160 đơn vị/ml–10%) là khoảng $665 cho lượng cung cấp 26,25 ml ($25,33/đơn vị). Mức giá này có thể thay đổi tùy thuộc vào nhà thuốc bạn đến hoặc phạm vi bảo hiểm y tế bạn được hưởng.
Hỗ trợ đồng thanh toán
Chắc chắn các chương trình cung cấp hỗ trợ với giá Hyqvia. Ưu đãi có thể ở nhiều hình thức khác nhau, bao gồm phiếu giảm giá in được, hoàn tiền, ưu đãi dùng thử, thẻ tiết kiệm hoặc mẫu dùng thử miễn phí. Một số ưu đãi có thể được in trực tiếp từ trang web. Một số khác yêu cầu đăng ký hợp lệ, hoàn thành bảng câu hỏi hoặc lấy mẫu từ văn phòng nhà cung cấp.
Các chương trình hỗ trợ đồng thanh toán bao gồm:
- Hyqvia Chương trình Hỗ trợ Đồng thanh toán OnePath
- Hyqvia HelloHyqvia Ưu đãi dùng thử miễn phí
- Chương trình đồng thanh toán của Quỹ HealthWell
- Quỹ Mạng lưới Tiếp cận Bệnh nhân (PAN)
- Chương trình Hỗ trợ Bệnh nhân OnePath (Hyqvia)
Ghi chú: Tất cả các chương trình nêu trên đều có yêu cầu về điều kiện tham gia khác nhau.
Nếu bạn muốn được giúp đỡ trong việc đăng ký bất kỳ chương trình nào trong số này, vui lòng liên hệ với chúng tôi để được hỗ trợ tài chính.
Nhận hỗ trợ đồng thanh toán Hyqvia
Hyqvia Hỗ trợ tài chínhHyqvia so với Hizentra
Giống như Hyqvia, Hizentra được chỉ định cho hội chứng suy giảm miễn dịch nguyên phát và bệnh lý viêm đa rễ thần kinh mất myelin mạn tính (CIDP).
Hizentra được sử dụng như liệu pháp thay thế cho bệnh suy giảm miễn dịch dịch thể nguyên phát (PI) ở người lớn và bệnh nhi từ 2 tuổi trở lên.
Hizentra cũng có thể được sử dụng cho các chẩn đoán khác. Liều tiêm dưới da Hizentra khoảng $209,01 cho lượng cung cấp 5 ml ($41,80/đơn vị).
Giá này có thể thay đổi tùy theo hiệu thuốc.
Ưu điểm của Hyqvia so với Hizentra:
- Chi phí thấp hơn trên mỗi ml – Tuy nhiên, điều quan trọng cần lưu ý là Hyqvia có nồng độ thấp hơn Hizentra (10% so với 20% IgG), và cần thể tích lớn hơn để điều trị. Do đó, tổng chi phí của Hyqvia thường tương đương với Hizentra trong nhiều trường hợp mặc dù chi phí trên mỗi ml thấp hơn.
- Liều dùng ít thường xuyên hơn (hàng tháng so với hai tuần một lần)
- 1 đến 2 vị trí truyền dịch
Nhược điểm của Hyqvia so với Hizentra:
- Phải truyền hai thành phần: Hy và Ig
- Thêm chất lỏng để truyền
- Thời gian truyền lâu hơn