Ilumya injection is FDA-approved to treat adults with moderate-to-severe plaque psoriasis. If you or anyone you love is living with plaque psoriasis, this article will cover everything you need to know about this medication, including its uses, mechanism, dosage, side effects, and cost.
مالي مرسته ترلاسه کړئ
Important Ilumya Warnings
Increased Risk of Infection
This medication may suppress your immune system, increasing the risk of infections. Before starting treatment, your healthcare provider will order tests to see if you have an infection or tuberculosis (TB).
If you have active TB or have had it in the past, your healthcare provider may treat you for TB before starting treatment.
Call your healthcare provider right away if you have the following signs of infection:
- Fever, sweats, or chills
- ټوخی
- د ساه لنډۍ
- Blood in your mucus
- د عضلاتو درد
- Warm, red, or painful skin or sores that aren’t due to psoriasis
- د وزن کمول
- نس ناستې
- د معدې درد
- Burning during urination
- Increased urine output
Before Taking Ilumya
Before you receive the first dose, inform your healthcare provider if you:
- Have a history of allergy to this product or any product components.
- Have long-term (chronic) or recurrent infection.
- Have TB, or someone close to you has been diagnosed with TB.
- Have recently been vaccinated or are scheduled to get vaccinated. You shouldn’t receive live vaccines during the treatment period. Live vaccine examples include MMR (measles, mumps, rubella), chickenpox, and nasal spray flu vaccines.
Ilumya Introduction and Uses
Ilumya is a brand-name prescription medication. Generic versions aren’t available.
The active ingredient in this product is tildrakizumab-asmn, which is in a category of targeted drug therapies called monoclonal antibodies.
Monoclonal antibodies are lab-created immune system proteins that can suppress, mimic, or enhance your immune response.
In March 2018, the US FDA approved this product to treat moderate-to-severe plaque psoriasis. Adults who may benefit from systemic therapy (oral or injectable medicine) or light therapy are considered for Ilumya therapy.
Plaque psoriasis is an د اتومیمون ناروغي characterized by thick, scaly patches on the elbows, back, knees, and scalp. In severe cases, the patches may affect the entire body.
همدا اوس د کاپي مرسته ترلاسه کړئ
Ilumya Mechanism of Action
Ilumya directly blocks the action of a protein, IL-23, which plays a crucial role in inflammatory and immune responses. By blocking IL-23, Ilumya:
- Helps balance an overactive immune response.
- Decreases the buildup of inflammatory cells in the skin.
- Stops the formation of scaly patches.
As a result, the skin becomes clearer, less inflamed, and less itchy. Clinical studies show that nearly 60% of individuals who use Ilumya have minimal or no symptoms after 3 months.
Ilumya Dosage
This medication comes as a clear to slightly yellow liquid to be injected under the skin (subcutaneous/SC).
The following strength is available in the US: 100 mg/ml solution in a single-dose prefilled syringe.
Your healthcare provider or a nurse administers Ilumya injection into the stomach area, thigh, or upper arm. The first dose is 100 mg SC. The second dose is 100 mg SC after 4 weeks. Then, 100 mg SC is administered every 12 weeks.
یادونه: You shouldn’t self-administer the Ilumya injection.
Ilumya Side Effects
عام جانبي عوارض
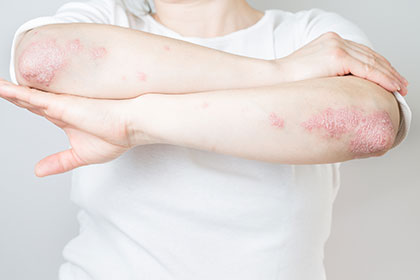
Call your healthcare provider if the following symptoms worsen or don’t go away.
- د پورتنۍ تنفسي لارې انتانات
- Injection site reactions (pain, swelling, or bleeding at the injection site)
- نس ناستې
الرجیک غبرګونونه
که تاسو تجربه کوئ نو بیړني طبي پاملرنې ته اړتیا لرئ:
- A feeling like you’re going to pass out
- Swelling of your face, eyelids, lips, mouth, tongue, or throat
- A skin rash
- تنفسي ستونزه
- Throat or chest tightness
د امیندوارۍ او شیدو ورکولو پرمهال کارول
Due to limited data regarding Ilumya use in pregnant women, it’s impossible to say if the drug will harm the unborn baby. Also, it’s unknown if this medication makes it harder for women to get pregnant.
Ilumya may pass from the mother to the unborn baby. However, the impact on the mother or unborn baby is unknown.
Animal reproduction studies haven’t reported a higher risk of miscarriage or birth defects with use during pregnancy [1].
No data are available about use during breastfeeding. Because tildrakizumab is a large protein, it’s unlikely to pass into breast milk. However, the medication was detected in breast milk in animal studies.
له لاسه ورکړل شوی خوراک
If you miss an appointment with your healthcare provider to receive your dose of Illumya, schedule another appointment as soon as possible.
ډیر خوراک
In overdose cases, seek emergency medical attention or call the poison help line at 1-800-222-1222.
له متخصص سره خبرې وکړئ
د کاپي مرستې په اړهIlumya Cost
1 unit (100 mg/ml SC solution) costs around $19,000.
However, the cost can vary depending on your insurance plan, location, and pharmacy. Contact your insurance provider to find out if your plan covers this medication or if you need prior authorization.
Sun Pharmaceutical Industries, Inc., the manufacturer of Ilumya, provides financial support if you’re eligible.
Those with commercial insurance may enroll in the ILUMYA SUPPORT LIGHTING THE WAY® program and pay $0 for each dose with the copay card.
For those without commercial insurance, ILUMYA SUPPORT LIGHTING THE WAY® may help by connecting them to a dedicated support specialist.
The cost of a prescription may be $0 for those who have Medicare Part B with supplemental insurance.
موږ سره اړیکه ونیسئ if you are interested in exploring financial assistance options for Ilumya.
Ilumya vs. Skyrizi
While both Ilumya and Skyrizi are used to treat moderate-to-severe plaque psoriasis, they differ in several ways. Below are some of the differences:
| د Ilumya معرفي کول | Skyrizi | |
| FDA approval date | 2018 | 2019 |
| Active drug | tildrakizumab | risankizumab |
| Who can administer? | Only a healthcare professional | Self-administration after training |
| Available strength | 100 mg | 75 mg |
| Approved uses | Moderate-to-severe plaque psoriasis in adults | Moderate-to-severe plaque psoriasis, active psoriatic arthritis, د کرون ناروغي, and ulcerative colitis in adults |
حوالې:
- MotherToBaby | Fact Sheets [Internet]. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); 1994-. Tildrakizumab (Ilumya®) 2023 Feb. Available from: https://www.ncbi.nlm.nih.gov/books/NBK582978/