Brand name: Hyqvia
Manufacturer: Baxter Healthcare Corporation, Baxter BioScience
Alternatives: Immune globulin infusion 10% with recombinant human hyaluronidase
Get Your Hyqvia Dose – Speak to a Specialist
What Is Hyqvia?
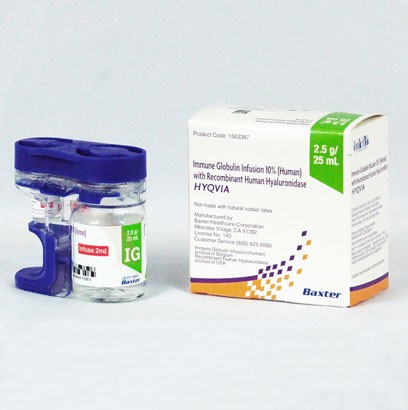
Hyqvia is an injectable drug containing a genetically designed protein. It is a dual vial unit with a vial of immune globulin infusion 10% (Ig) and another vial of recombinant human hyaluronidase (Hy), which assists your body in the absorption of injectable medicines.
The parts are infused one after the other (Hy first, Ig second) through a needle set.
Treatment/Therapy
Hyqvia is an immune globulin infusion. It is a sterilized solution made up of human plasma containing antibodies that help your body protect itself against different infectious diseases. It is used in treating primary immunodeficiency in adults.
Infusion
Hyqvia is administered in the form of a subcutaneous infusion, given under the skin. It has flexible administration options, and a healthcare provider can teach you how to self-administer in the comfort of your own home.
Do not self-administer the medication if you do not understand the instructions properly. The infusion can also be administered at an infusion center.
Storage
- Hyqvia can be stored in a refrigerator at 36°F to 46°F for up to 3 years (do not freeze the medication).
- It may be stored at room temperature (up to 77°F) for up to 3 months, but only during the first 24 months from the date of manufacture printed on the carton.
- Do not return Hyqvia to the refrigerator if you take it out to store at room temperature.
- Store Hyqvia in its carton to protect it from light.
- The refrigerated product should be allowed to reach room temperature before use.
- Do not heat or microwave the product.
Administration
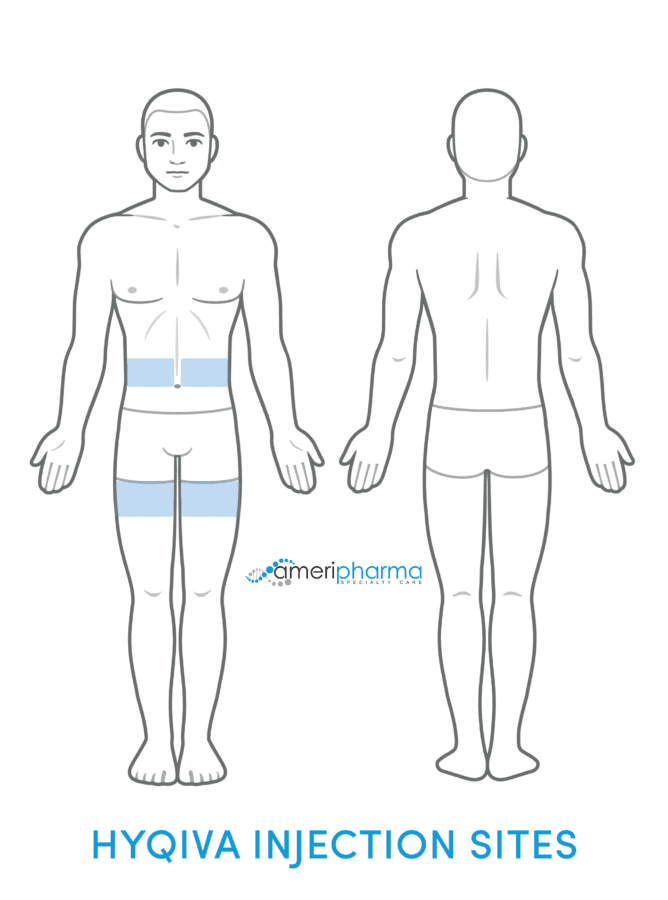
For infusion, an infusion pump is needed to titrate the appropriate flow rate. To prevent leakage at the infusion site while administering Hyqvia, a longer needle (12 or 14 mm) and the use of multiple infusion sites should be considered.
- Before infusing, inspect the vials for any cloudy precipitates, and do not shake the vials.
- Do not use the medication if the solution is not clear.
- Each vial is single-use and should not be used more than once.
- Suggested infusion sites are the thighs or abdomen.
- When infusing, do not mix the two medications given in the Hyqvia kit.
Sequential Infusion
Both of the components of Hyqvia are infused sequentially, starting first with Hyaluronidase, then Ig 10% within 10 minutes through the same subcutaneous needle.
The entire contents of the Hyaluronidase vial are used for each full or partial vial of Ig.
Local infusion-site reactions such as mild to moderate pain, temporary soft pancake-like swelling, itching, and skin redness are common.
Swelling may last 1 to 3 days, and infusion-related reactions should go away in a few hours.
Hyqvia Copay Assistance Available
Contraindications
Do not use Hyqvia If you ever had an allergic reaction to immune globulin, human albumin, and hyaluronidase or if you are immunoglobulin A deficient.
Warnings and Precautions
Hyqvia can cause blood clots, especially if you are 65 years or older, have heart disease, circulation disease, or a history of blood clots.
Immediately contact your provider if you experience sudden chest pain, numbness, slurred speech, vision disorder, shortness of breath or pain, warmth, or swelling in the leg or arm.
Inform your health care professional before administering infusion if you have any other health conditions such as diabetes, kidney disease, pregnancy, or dehydration. Do not infuse into or around an infected area as it may cause the infection to spread.
Drug Interactions
Hyqvia may interact and interfere with the immune responses to live virus vaccines, such as those for measles, mumps, rubella (MMR), and chickenpox.
Side Effects
According to clinical trials, more than 5% of the subjects experienced some common side effects, including headache, fever, vomiting, nausea, fatigue, antibody formation against hyaluronidase, and symptoms of allergy such as swelling, itching, pain, and skin rash. There is no evidence of skin damage from Hyqvia so far.
Hyqvia can cause blood clots or thrombosis. Get immediate medical assistance if you experience any of the following adverse effects:
- Speech and vision problems
- Sudden numbness and lethargy
- Chest pain
- Blood in cough
- Dark urine
- Decreased urination
- Jaundice
- Infection
- Fast heartbeat
- Blood clotting in arm or leg
- Pulmonary disease such as transfusion-related acute lung injury (TRALI)
Home Infusion Available
Dosing
The dosing of Hyqvia is on an individualized basis.
Hyqvia dosing variations are discussed below:
- If the patient already takes Ig infusions, the treatment should be initiated 1 week after the last Ig infusion.
- Gradually increase dosage and frequency from a 1-week dose to a 3 or 4-week dose.
- The initial dose is 7.5 g on week 1, 15 g on week 2, 22.5 g on week 4, then 30 g on week 7 (full information is available in the Hyqvia package insert).
- If the patient has switched from IVIG treatment, then administer Hyqvia at the same dose and frequency as the previous IV treatment after initial dose ramp-up.
- If the patient has switched from SCIG treatment or is IgG-naïve, then administer at 300 – 600 mg/kg at 3 to 4 week intervals after initial ramp-up.
- If the patient is at high risk of renal failure or thrombosis, then administer Hyqvia at the minimum dose and infusion rate practicable.
Volume Per Site:
Up to 600 ml per site can be administered for patients who weigh 40 kg or more and up to 300 ml per site can be administered for those who weigh less than 40 kg.
Rate of Infusion:
Administer the hyaluronidase of Hyqvia (HY) at an initial rate per site of approximately 1 to 2 ml per minute or as tolerated.
Administer the immune globulin 10% (IG) component of Hyqvia at the rates shown below for the initial infusions. Based on tolerability, these rates may be adjusted by the pharmacist.
| First 2 Infusions | Subsequent 2 or 3 Infusions | |||
|---|---|---|---|---|
| Subjects < 40 kg (< 88lbs) |
Subjects ≥ 40 kg (≥ 88lbs) |
Subjects < 40 kg (< 88lbs) |
Subjects ≥ 40 kg (≥ 88lbs) |
|
| Intervals (minutes) |
Rate per site (ml per hour) |
Rate per site (ml per hour) |
Rate per site (ml per hour) |
Rate per site (ml per hour) |
| 5 – 15 | 5 | 10 | 10 | 10 |
| 5 – 15 | 10 | 30 | 20 | 30 |
| 5 – 15 | 20 | 60 | 40 | 120 |
| 5 – 15 | 40 | 120 | 80 | 240 |
| Remainder of infusion | 80 | 240 | 160 | 300 |
Uses
Hyqvia is mainly indicated for treating primary immunodeficiency (PI) in adults.
Immunodeficiency conditions may include common variable immunodeficiency, Wiskott-Aldrich syndrome, congenital or X-linked agammaglobulinemia, and severe combined immunodeficiencies.
Hyqvia Cost
The average cost for Hyqvia subcutaneous solution (160 units/ml-10%) is approximately $567 for a supply of 26.25 ml ($21.59/unit). This price may vary depending on the pharmacy.
Copay Assistance
Certain programs offer assistance with the cost of Hyqvia. Offers may be in different forms, including a printable coupon, rebate, trial offer, savings card, or free samples. Some offers may be printed directly from a website. Others require proper enrollment, completing a questionnaire, or obtaining a sample from the provider’s office.
Copay assistance programs include:
- Hyqvia OnePath Co-Pay Assistance Program
- Hyqvia HelloHyqvia Free Trial Offer
- HealthWell Foundation Copay Program
- Patient Access Network Foundation (PAN)
- OnePath Patient Assistance Program (Hyqvia)
Note: All the above-mentioned programs have different eligibility requirements.
Get Hyqvia Copay Assistance | Hyqvia Financial Assistance
Hyqvia vs. Hizentra
Hizentra is indicated for primary immunodeficiency syndrome and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
Hizentra is used as replacement therapy for primary humoral immunodeficiency (PI) in adults and pediatric patients 2 years and older.
Hizentra may also be used for other diagnoses. Hizentra subcutaneous injection is approximately $209.01 for a supply of 5 ml ($41.80/unit).
This price may vary depending on the pharmacy.
Pros of Hyqvia vs. Hizentra:
- Lower cost per ml
- Infused every 3 to 4 weeks
- 1 to 2 infusion sites
Cons of Hyqvia vs. Hizentra:
- Only FDA-approved to treat primary immunodeficiency
- Must infuse two components: Hy and Ig
- More fluid to infuse
- Longer infusion time
Contact Us
By submitting, you agree to AmeriPharma’s Terms of Use, Privacy Policy, and Notice of Privacy Practice.
This information is not a substitute for medical advice or treatment. Talk to your doctor or healthcare provider about your medical condition prior to starting any new treatment. AmeriPharma Specialty Care assumes no liability whatsoever for the information provided or for any diagnosis or treatment made as a result, nor is it responsible for the reliability of the content.
AmeriPharma Specialty Care does not operate all the websites/organizations listed here, nor is it responsible for the availability or reliability of their content. These listings do not imply or constitute an endorsement, sponsorship, or recommendation by AmeriPharma Specialty Care.
This webpage may contain references to brand-name prescription drugs that are trademarks or registered trademarks of pharmaceutical manufacturers not affiliated with AmeriPharma Specialty Care.

Dr. Christine Leduc, PharmD, was born and raised in Irvine, CA. She attended college at Midwestern University, where she graduated cum laude. The most rewarding part of her job is suggesting lifestyle changes, educating patients on how their medication works, and precepting future pharmacists. Her areas of expertise are customer service and knowledge of specialty medication. Having worked in the service industry in the past, she has gained the customer service skills necessary to understand the needs of her patients. Dr. Leduc is currently precepting students from Marshall B. Ketchum University, University of Kansas, and Midwestern University. In her free time, she enjoys traveling, baking, and gardening.